Особенности сплайсинг-ориентированных ДНК-микрочипов и их применение в биомедицинских исследованиях (обзор)
Альтернативный сплайсинг (АС) обеспечивает многообразие изоформ белков и зрелых мРНК, относящихся к одному гену, и является необходимым звеном в ходе дифференцировки и функционирования клеток и тканей. ДНК-микрочипы — высокопроизводительный метод изучения транскриптома как на уровне суммарной экспрессии генов, так и на уровне репертуара альтернативно-сплайсированных изоформ мРНК. Изучение паттернов АС обусловливает необходимость тщательных процедур подбора последовательностей зондов для обеспечения надлежащей точности анализа.
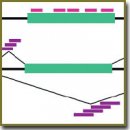
Существует два основных типа ДНК-микрочипов, ориентированных на изучение АС. Микрочипы первого типа содержат зонды, направленные на участки внутри границ экзонов (тела экзонов); микрочипы второго типа содержат зонды, направленные как на тела экзонов, так и на участки соединений экзон–экзон, экзон–интрон. Рассмотрены особенности подбора последовательностей зондов и общий дизайн двух типов ДНК-микрочипов, охарактеризованы их основные преимущества и ограничения.
Отдельный раздел посвящен результатам исследований АС, полученным с применением ДНК-микрочипов. В частности, с применением ДНК-микрочипов был выявлен ряд механизмов процессинга и сплайсинга пре-мРНК, описаны паттерны АС, ассоциированные с онкологическими заболеваниями, процессами дифференцировки клеток и тканей. Показано, что регуляция аппарата сплайсинга является необходимой составляющей в ходе канцерогенеза и дифференцировки. Рассмотрены примеры использования сплайсинг-ориентированных ДНК-микрочипов при выявлении диагностических маркеров и механизмов развития патологий. Перспективным направлением исследований является изучение роли и механизмов АС при дифференцировке и поддержании плюрипотентного состояния индуцированных стволовых клеток, функционировании иммуноцитов и инфицированных клеток в ходе иммунного ответа на инфекцию. Сплайсинг-ориентированные ДНК-микрочипы являются сравнительно недорогим, но информативным инструментом исследований, что дает основание предполагать их внедрение в клиническую практику в течение ближайших лет.
- Wang E.T., Sandberg R., Luo S., Khrebtukova I., Zhang L., Mayr C., Kingsmore S.F., Schroth G.P., Burge C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008; 456(7221): 470–476, http://dx.doi.org/10.1038/nature07509.
- Pan Q., Shai O., Lee L.J., Frey B.J., Blencowe B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high throughput sequencing. Nat Genet 2008; 40(12): 1413–1435, http://dx.doi.org/10.1038/ng.259.
- Novais R.C., Thorstenson Y.R. The evolution of Pyrosequencing® for microbiology: from genes to genomes. J Microbiol Methods 2011; 86(1): 1–7, http://dx.doi.org/10.1016/j.mimet.2011.04.006.
- Wang Z., Gerstein M., Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 2009; 10(1): 57–63, http://dx.doi.org/10.1038/nrg2484.
- Ramani A.K., Calarco J.A., Pan Q., Mavandadi S., Wang Y., Nelson A.C., Lee L.J., Morris Q., Blencowe B.J., Zhen M., Fraser A.G. Genome-wide analysis of alternative splicing in Caenorhabditis elegans. Genome Res 2011; 21(2): 342–348, http://dx.doi.org/10.1101/gr.114645.110.
- Malone J.H., Oliver B. Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biology 2011; 9: 34, http://dx.doi.org/10.1186/1741-7007-9-34.
- RNA-Seq misses what HTA delivers. URL: http://www.affymetrix.com/fa/media/hta_array_2_0_flyer.pdf.
- Affymetrix. GeneChip® Exon Array design. URL: http://media.affymetrix.com/support/technical/technotes/exon_array_design_technote.pdf.
- Affymetrix. GeneChip® Exon Array System for human, mouse, and rat. URL: http://www.affymetrix.com/support/technical/datasheets/exon_arraydesign_datasheet.pdf.
- Jaksik R., Marczyk M., Polanska J., Rzeszowska-Wolny J. Sources of high variance between probe signals in Affymetrix short oligonucleotide microarrays. Sensors (Basel) 2014; 14(1): 532–548; http://dx.doi.org/10.3390/s140100532.
- Hung L.H., Heiner M., Hui J., Schreiner S., Benes V., Bindereif A. Diverse roles of hnRNP L in mammalian mRNA processing: a combined microarray and RNAi analysis. RNA 2008; 14(2): 284–296, http://dx.doi.org/10.1261/rna.725208.
- Kurokawa K., Akaike Y., Masuda K., Kuwano Y., Nishida K., Yamagishi N., Kajita K., Tanahashi T., Rokutan K. Downregulation of serine/arginine-rich splicing factor 3 induces G1 cell cycle arrest and apoptosis in colon cancer cells. Oncogene 2014; 33(11): 1407–1417, http://dx.doi.org/10.1038/onc.2013.86.
- Yamashita Y., Matsuura T., Shinmi J., Amakusa Y., Masuda A., Ito M., Kinoshita M., Furuya H., Abe K., Ibi T., Sahashi K., Ohno K. Four parameters increase the sensitivity and specificity of the exon array analysis and disclose 25 novel aberrantly spliced exons in myotonic dystrophy. J Hum Genet 2012; 57(6): 368–374, http://dx.doi.org/10.1038/jhg.2012.37.
- Clark T.A., Schweitzer A.C., Chen T.X., Staples M.K., Lu G., Wang H., Williams A., Blume J.E. Discovery of tissue specific exons using comprehensive human exon microarrays. Genome Biol 2007; 8(4): R64, http://dx.doi.org/10.1186/gb-2007-8-4-r64.
- Xing Y., Stoilov P., Kapur K., Han A., Jiang H., Shen S., Black D.L., Wong W.H. MADS: a new and improved method for analysis of differential alternative splicing by exon-tiling microarrays. RNA 2008; 14(8): 1470–1479, http://dx.doi.org/10.1261/rna.1070208.
- Risueño A., Roson-Burgo B., Dolnik A., Hernandez-Rivas J.M., Bullinger L., De Las Rivas J. A robust estimation of exon expression to identify alternative spliced genes applied to human tissues and cancer samples. BMC Genomics 2014; 15: 879, http://dx.doi.org/10.1186/1471-2164-15-879.
- Chen P., Lepikhova T., Hu Y., Monni O., Hautaniemi S. Comprehensive exon array data processing method for quantitative analysis of alternative spliced variants. Nucleic Acids Research 2011; 39(18): e123, http://dx.doi.org/10.1093/nar/gkr513.
- Kapetis D., Clarelli F., Vitulli F., de Rosbo N.K., Beretta O., Foti M., Ricciardi-Castagnoli P., Zolezzi F. AMDA 2.13: a major update for automated cross-platform microarray data analysis. Biotechniques 2012; 53(1): 33–40.
- Liu X., Gao Z., Zhang L., Rattray M. puma 3.0: improved uncertainty propagation methods for gene and transcript expression analysis. BMC Bioinformatics 2013; 14: 39, http://dx.doi.org/10.1186/1471-2105-14-39.
- Agilent Technologies. Comprehensive coverage with the Agilent SurePrint G3 Exon Microarray system. URL: http://www.agilent.com/cs/library/brochures/5990-6928en_lo.pdf.
- Hu X., Wu R., Shehadeh L.A., Zhou Q., Jiang C., Huang X., Zhang L., Gao F., Liu X., Yu H., Webster K.A., Wang J. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genomics 2014; 15: 303, http://dx.doi.org/10.1186/1471-2164-15-303.
- Pesson M., Eymin B., De La Grange P., Simon B., Corcos L. A dedicated microarray for in-depth analysis of pre-mRNA splicing events: application to the study of genes involved in the response to targeted anticancer therapies. Molecular Cancer 2014, 13: 9, http://dx.doi.org/10.1186/1476-4598-13-9.
- Li C., Kato M., Shiue L., Shively J.E., Ares M. Jr., Lin R.J. Cell type and culture condition-dependent alternative splicing in human breast cancer cells revealed by splicing-sensitive microarrays. Cancer Res 2006; 66(4): 1990–1999, http://dx.doi.org/10.1158/0008-5472.can-05-2593.
- Zhou W., Calciano M.A., Jordan H., Brenner M., Johnson S., Wu D., Lei L., Pallares D., Beurdeley P., Rouet F., Gill P.S., Bracco L., Soucaille C., Einstein R. High resolution analysis of the human transcriptome: detection of extensive alternative splicing independent of transcriptional activity. BMC Genet 2009; 10: 63, http://dx.doi.org/10.1186/1471-2156-10-63.
- Srinivasan K., Shiue L., Hayes J.D., Centers R., Fitzwater S., Loewen R., Edmondson L.R., Bryant J., Smith M., Rommelfanger C., Welch V., Clark T.A., Sugnet C.W., Howe K.J., Mandel-Gutfreund Y., Ares M. Jr. Detection and measurement of alternative splicing using splicing-sensitive microarrays. Methods 2005; 37: 345–359, http://dx.doi.org/10.1016/j.ymeth.2005.09.007.
- Fehlbaum P., Guihal C., Bracco L., Cochet O. A microarray configuration to quantify expression levels and relative abundance of splice variants. Nucleic Acids Res 2005; 33(5): e47, http://dx.doi.org/10.1093/nar/gni047.
- Harrison A., Binder H., Buhot A., Burden C.J., Carlon E., Gibas C., Gamble L.J., Halperin A., Hooyberghs J., Kreil D.P., Levicky R., Noble P.A., Ott A., Pettitt B.M., Tautz D., Pozhitkov A.E. Physico-chemical foundations underpinning microarray and next-generation sequencing experiments. Nucleic Acids Res 2013; 41(5): 2779–2796, http://dx.doi.org/10.1093/nar/gks1358.
- Affymetrix. GeneChip® WT PLUS Reagent Kit. URL: http://media.affymetrix.com/support/downloads/manuals/wtplus_reagentkit_assay_manual.pdf.
- Agilent 2015. Agilent One-Color Microarray-Based Exon Analysis — Low Input Quick Amp WT Labeling. URL: http://www.agilent.com/cs/library/usermanuals/Public/G4140-90042_Exon_One-color_2.0.pdf.
- Castle J.C., Zhang C., Shah J.K., Kulkarni A.V., Kalsotra A., Cooper T.A., Johnson J.M. Expression of 24,426 human alternative splicing events and predicted cis regulation in 48 tissues and cell lines. Nat Gen 2008; 40(12): 1416–1425, http://dx.doi.org/10.1038/ng.264.
- Muñoz M.J., Pérez Santangelo M.S., Paronetto M.P., de la Mata M., Pelisch F., Boireau S., Glover-Cutter K., Ben-Dov C., Blaustein M., Lozano J.J., Bird G., Bentley D., Bertrand E., Kornblihtt A.R. DNA damage regulates alternative splicing through inhibition of RNA polymerase II elongation. Cell 2009; 137(4): 708–720, http://dx.doi.org/10.1016/j.cell.2009.03.010.
- Paronetto M.P., Miñana B., Valcarcel J. The Ewing sarcoma protein regulates DNA damage-induced alternative splicing. Mol Cell 2011; 43(3): 353–368, http://dx.doi.org/10.1016/j.molcel.2011.05.035.
- Bava F.A., Eliscovich C., Ferreira P.G., Miсana B., Ben-Dov C., Guigó R., Valcárcel J., Méndez R. CPEB1 coordinates alternative 3’-UTR formation with translational regulation. Nature 2013; 495(7429): 121–125, http://dx.doi.org/10.1038/nature11901.
- Le K., Mitsouras K., Roy M., Wang Q., Xu Q., Nelson S.F., Lee C. Detecting tissue-specific regulation of alternative splicing as a qualitative change in microarray data. Nucleic Acids Res 2004; 32(22): e180, http://dx.doi.org/10.1093/nar/gnh173.
- Xu W., Seok J., Mindrinos M.N., Schweitzer A.C., Jiang H., Wilhelmy J., Clark T.A., Kapur K., Xing Y., Faham M., Storey J.D., Moldawer L.L., Maier R.V., Tompkins R.G., Wong W.H., Davis R.W., Xiao W.; Inflammation and Host Response to Injury Large-Scale Collaborative Research Program. Human transcriptome array for high-throughput clinical studies. Proc Natl Acad Sci USA 2011; 108(9): 3707–3712, http://dx.doi.org/10.1073/pnas.1019753108.
- Corrionero A., Miñana B., Valcárcel J. Reduced fidelity of branch point recognition and alternative splicing induced by the anti-tumor drug spliceostatin A. Gen Dev 2011; 25(5): 445–459, http://dx.doi.org/10.1101/gad.2014311.
- Solier S., Barb J., Zeeberg B.R., Varma S., Ryan M.C., Kohn K.W., Weinstein J.N., Munson P.J., Pommier Y. Genome-wide analysis of novel splice variants induced by topoisomerase I poisoning shows preferential occurrence in genes encoding splicing factors. Cancer Res 2010; 70(20): 8055–8065, http://dx.doi.org/10.1158/0008-5472.CAN-10-2491.
- Solier S., Ryan M.C., Martin S.E., Varma S., Kohn K.W., Liu H., Zeeberg B.R., Pommier Y. Transcription poisoning by Topoisomerase I is controlled by gene length, splice sites, and miR-142-3p. Cancer Res 2013; 73(15): 4830–4839, http://dx.doi.org/10.1158/0008-5472.CAN-12-3504.
- Dutertre M., Chakrama F.Z., Combe E., Desmet F.O., Mortada H., Polay Espinoza M., Gratadou L., Auboeuf D. A recently evolved class of alternative 3’-terminal exons involved in cell cycle regulation by topoisomerase inhibitors. Nat Commun 2014; 5: 3395, http://dx.doi.org/10.1038/ncomms4395.
- Liu Z.-R. p68 RNA helicase is an essential human splicing factor that acts at the U1 snRNA-5’ splice site duplex. Mol Cell Biol 2002; 22(15): 5443–5450, http://dx.doi.org/10.1128/mcb.22.15.5443-5450.2002.
- Dardenne E., Polay Espinoza M., Fattet L., Germann S., Lambert M.P., Neil H., Zonta E., Mortada H., Gratadou L., Deygas M., Chakrama F.Z., Samaan S., Desmet F.O., Tranchevent L.C., Dutertre M., Rimokh R., Bourgeois C.F., Auboeuf D. RNA helicases DDX5 and DDX17 dynamically orchestrate transcription, miRNA, and splicing programs in cell differentiation. Cell Rep 2014; 7(6): 1900–1913, http://dx.doi.org/10.1016/j.celrep.2014.05.010.
- Samaan S., Tranchevent L.-C., Dardenne E., Espinoza M.P., Zonta E., Germann S., Gratadou L., Dutertre M., Auboeuf D. The Ddx5 and Ddx17 RNA helicases are cornerstones in the complex regulatory array of steroid hormone-signaling pathways. Nucleic Acids Res 2014; 42(4): 2197–2207, http://dx.doi.org/10.1093/nar/gkt1216.
- Pandit S., Zhou Y., Shiue L., Coutinho-Mansfield G., Li H., Qiu J., Huang J., Yeo G.W., Ares M., Fu X.-D. Genome-wide analysis reveals SR protein cooperation and competition in regulated splicing. Mol Cell 2013; 50(2): 223–235, http://dx.doi.org/10.1016/j.molcel.2013.03.001.
- Huelga S.C., Vu A.Q., Arnold J.D., Liang T.Y., Liu P.P., Yan B.Y., Donohue J.P., Shiue L., Hoon S., Brenner S., Ares M. Jr., Yeo G.W. Integrative genome-wide analysis reveals cooperative regulation of alternative splicing by hnRNP proteins. Cell Rep 2012; 1(2): 167–178, http://dx.doi.org/10.1016/j.celrep.2012.02.001.
- Llorian M., Schwartz S., Clark T.A., Hollander D., Tan L.Y., Spellman R., Gordon A., Schweitzer A.C., de la Grange P., Ast G., Smith C.W. Position-dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nat Struct Mol Biol 2010; 17(9): 1114–1123, http://dx.doi.org/10.1038/nsmb.1881.
- Xi L., Feber A., Gupta V., Wu M., Bergemann A.D., Landreneau R.J., Litle V.R., Pennathur A., Luketich J.D., Godfrey T.E. Whole genome exon arrays identify differential expression of alternatively spliced, cancer-related genes in lung cancer. Nucleic Acids Res 2008; 36(20): 6535–6547, http://dx.doi.org/10.1093/nar/gkn697.
- de Miguel F.J., Sharma R.D., Pajares M.J., Montuenga L.M., Rubio A., Pio R. Identification of alternative splicing events regulated by the oncogenic factor SRSF1 in lung cancer. Cancer Res 2014; 74(4): 1105–1115, http://dx.doi.org/10.1158/0008-5472.CAN-13-1481.
- Gardina P.J., Clark T.A., Shimada B., Staples M.K., Yang Q., Veitch J., Schweitzer A., Awad T., Sugnet C., Dee S., Davies C., Williams A., Turpaz Y. Alternative splicing and differential gene expression in colon cancer detected by a whole genome exon array. BMC Genomics 2006; 7(1): 325, http://dx.doi.org/10.1186/1471-2164-7-325.
- Guo X., Chen Q.R., Song Y.K., Wei J.S., Khan J. Exon array analysis reveals neuroblastoma tumors have distinct alternative splicing patterns according to stage and MYCN amplification status. BMC Med Genomics 2011; 4: 35, http://dx.doi.org/10.1186/1755-8794-4-35.
- Yu F., Fu W.-M. Identification of differential splicing genes in gliomas using exon expression profiling. Mol Med Rep 2015; 11(2): 843–850, http://dx.doi.org/10.3892/mmr.2014.2775.
- Gerber J.M., Gucwa J.L., Esopi D., Gurel M., Haffner M.C., Vala M., Nelson W.G., Jones R.J., Yegnasubramanian S. Genome-wide comparison of the transcriptomes of highly enriched normal and chronic myeloid leukemia stem and progenitor cell populations. Oncotarget 2013; 4(5): 715–728, http://dx.doi.org/10.18632/oncotarget.990.
- Adamia S., Haibe-Kains B., Pilarski P.M., Bar-Natan M., Pevzner S., Avet-Loiseau H., Lode L., Verselis S., Fox E.A., Burke J., Galinsky I., Dagogo-Jack I., Wadleigh M., Steensma D.P., Motyckova G., Deangelo D.J., Quackenbush J., Stone R., Griffin J.D. A genome-wide aberrant RNA splicing in patients with acute myeloid leukemia identifies novel potential disease markers and therapeutic targets. Clin Cancer Res 2014; 20(5): 1135–1145, http://dx.doi.org/10.1158/1078-0432.CCR-13-0956.
- Adamia S., Bar-Natan M., Haibe-Kains B., Pilarski P.M., Bach C., Pevzner S., Calimeri T., Avet-Loiseau H., Lode L., Verselis S., Fox E.A., Galinsky I., Mathews S., Dagogo-Jack I., Wadleigh M., Steensma D.P., Motyckova G., Deangelo D.J., Quackenbush J., Tenen D.G., Stone R.M., Griffin J.D. NOTCH2 and FLT3 gene mis-splicings are common events in patients with acute myeloid leukemia (AML): new potential targets in AML. Blood 2014; 123(18): 2816–2825, http://dx.doi.org/10.1182/blood-2013-02-481507.
- Relógio A., Ben-Dov C., Baum M., Ruggiu M., Gemund C., Benes V., Darnell R.B., Valcárcel J. Alternative splicing microarrays reveal functional expression of neuron-specific regulators in Hodgkin lymphoma cells. J Biol Chem 2005; 280(6): 4779–4784, http://dx.doi.org/10.1074/jbc.m411976200.
- Li R., Ochs M.F., Ahn S.M., Hennessey P., Tan M., Soudry E., Gaykalova D.A., Uemura M., Brait M., Shao C., Westra W., Bishop J., Fertig E.J., Califano J.A. Expression microarray analysis reveals alternative splicing of LAMA3 and DST genes in head and neck squamous cell carcinoma. PLoS One 2014; 9(3): e91263, http://dx.doi.org/10.1371/journal.pone.0091263.
- Liu J., Xiao Y., Xiong H.-M., Li J., Huang B., Zhang H.-B., Feng D.-Q., Chen X.-M., Wang X.-Z. Alternative splicing of apoptosis-related genes in imatinib-treated K562 cells identified by exon array analysis. Int J Mol Med 2012; 29(4): 690–698, http://dx.doi.org/10.3892/ijmm.2011.872.
- Sveen A., Еgesen T.H., Nesbakken A., Rognum T.O., Lothe R.A., Skotheim R.I. Transcriptome instability in colorectal cancer identified by exon microarray analyses: Associations with splicing factor expression levels and patient survival. Genome Med 2011; 3(5): 32, http://dx.doi.org/10.1186/gm248.
- Sveen A., Johannessen B., Teixeira M.R., Lothe R.A., Skotheim R.I. Transcriptome instability as a molecular pan-cancer characteristic of carcinomas. BMC Genomics 2014; 15: 672, http://dx.doi.org/10.1186/1471-2164-15-672.
- Carrigan P.E., Bingham J.L., Srinvasan S., Brentnall T.A., Miller L.J. Characterization of alternative spliceoforms and the RNA splicing machinery in pancreatic cancer. Pancreas 2011; 40(2): 281–288, http://dx.doi.org/10.1097/MPA.0b013e31820128d2.
- Katiyar S., Casimiro M.C., Dettin L., Ju X., Wagner E.F., Tanaka H., Pestell R.G. C-jun inhibits mammary apoptosis in vivo. Mol Biol Cell 2010; 21(23): 4264–4274, http://dx.doi.org/10.1091/mbc.E10-08-0705.
- Albanese C., Johnson J., Watanabe G., Eklund N., Vu D., Arnold A., Pestell R.G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 1995; 270(40): 23589–23597, http://dx.doi.org/10.1074/jbc.270.40.23589.
- Eferl R., Ricci R., Kenner L., Zenz R., David J.P., Rath M., Wagner E.F. Liver tumor development: c-Jun antagonizes the proapoptotic activity of p53. Cell 2003; 112(2): 181–192, http://dx.doi.org/10.1016/s0092-8674(03)00042-4.
- Shaulian E., Schreiber M., Piu F., Beeche M., Wagner E.F., Karin M. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell 2000; 103(6): 897–907, http://dx.doi.org/10.1016/s0092-8674(00)00193-8.
- Katiyar S., Jiao X., Addya S., Ertel A., Covarrubias Y., Rose V., Casimiro M.C., Zhou J., Lisanti M.P., Nasim T., Fortina P., Pestell R.G. Mammary gland selective excision of c-Jun identifies its role in mRNA splicing. Cancer Res 2012; 72(4): 1023–1034, http://dx.doi.org/10.1158/0008-5472.CAN-11-3647.
- Lapuk A., Marr H., Jakkula L., Pedro H., Bhattacharya S., Purdom E., Hu Z., Simpson K., Pachter L., Durinck S., Wang N., Parvin B., Fontenay G., Speed T., Garbe J., Stampfer M., Bayandorian H., Dorton S., Clark T.A., Schweitzer A., Wyrobek A., Feiler H., Spellman P., Conboy J., Gray J.W. Exon-level microarray analyses identify alternative splicing programs in breast cancer. Mol Cancer Res 2010; 8(7): 961–974, http://dx.doi.org/10.1158/1541-7786.MCR-09-0528.
- Chen M., Manley J.L. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol 2009; 10: 741–754, http://dx.doi.org/10.1038/nrm2777.
- Hartmann B., Valcárcel J. Decrypting the genome’s alternative messages. Curr Opin Cell Biol 2009; 21(3): 377–386, http://dx.doi.org/10.1016/j.ceb.2009.02.006.
- Nilsen T.W., Graveley B.R. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010; 463(7280): 457–463, http://dx.doi.org/10.1038/nature08909.
- Warzecha C.C., Sato T.K., Nabet B., Hogenesch J.B., Carstens R.P. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell 2009; 33(5): 591–601, http://dx.doi.org/10.1016/j.molcel.2009.01.025.
- Warzecha C.C., Shen S., Xing Y., Carstens R.P. The epithelial splicing factors ESRP1 and ESRP2 positively and negatively regulate diverse types of alternative splicing events. RNA Biol 2009; 6(5): 546–562, http://dx.doi.org/10.4161/rna.6.5.9606.
- Warzecha C.C., Jiang P., Amirikian K., Dittmar K.A., Lu H., Shen S., Guo W., Xing Y., Carstens R.P. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J 2010; 29(19): 3286–3300, http://dx.doi.org/10.1038/emboj.2010.195.
- Das D., Clark T.A., Schweitzer A., Yamamoto M., Marr H., Arribere J., Minovitsky S., Poliakov A., Dubchak I., Blume J.E., Conboy J.G. A correlation with exon expression approach to identify cis-regulatory elements for tissue-specific alternative splicing. Nucleic Acids Res 2007; 35(14): 4845–4857, http://dx.doi.org/10.1093/nar/gkm485.
- Zhang C., Zhang Z., Castle J., Sun S., Johnson J., Krainer A.R., Zhang M.Q. Defining the regulatory network of the tissue-specific splicing factors Fox-1 and Fox-2. Genes Dev 2008; 22(18): 2550–2563, http://dx.doi.org/10.1101/gad.1703108.
- Boutz P.L., Stoilov P., Li Q., Lin C.H., Chawla G., Ostrow K., Shiue L., Ares M. Jr., Black D.L. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev 2007; 21(13): 1636–1652, http://dx.doi.org/10.1101/gad.1558107.
- Zhang C., Frias M.A., Mele A., Ruggiu M., Eom T., Marney C.B., Wang H., Licatalosi D.D., Fak J.J., Darnell R.B. Integrative modeling defines the Nova splicing-regulatory network and its combinatorial controls. Science 2010; 329(5990): 439–443, http://dx.doi.org/10.1126/science.1191150.
- Mallinjoud P., Villemin J.P., Mortada H., Polay Espinoza M., Desmet F.O., Samaan S., Chautard E., Tranchevent L.C., Auboeuf D. Endothelial, epithelial, and fibroblast cells exhibit specific splicing programs independently of their tissue of origin. Genome Res 2014; 24(3): 511–521, http://dx.doi.org/10.1101/gr.162933.113.
- Potashkin J.A., Santiago J.A., Ravina B.M., Watts A., Leontovich A.A. Biosignatures for Parkinson’s disease and atypical parkinsonian disorders patients. PLoS One 2012; 7(8): e43595, http://dx.doi.org/10.1371/journal.pone.0043595.
- Calciano M., Lemarié J.C., Blondiaux E., Einstein R., Fehlbaum-Beurdeley P. A predictive microarray-based biomarker for early detection of Alzheimer’s disease intended for clinical diagnostic application. Biomarkers 2013; 18(3): 264–272, http://dx.doi.org/10.3109/1354750X.2013.773083.
- Lai M.K.P., Esiri M.M., Tan M.G.K. Genome-wide profiling of alternative splicing in Alzheimer’s disease. Genomics Data 2014; 2: 290–292, http://dx.doi.org/10.1016/j.gdata.2014.09.002.
- Tollervey J.R., Wang Z., Hortobagyi T., Witten J.T., Zarnack K., Kayikci M., Clark T.A., Schweitzer A.C., Rot G., Curk T., Zupan B., Rogelj B., Shaw C.E., Ule J. Analysis of alternative splicing associated with aging and neurodegeneration in the human brain. Genome Res 2011; 21(10): 1572–1582, http://dx.doi.org/10.1101/gr.122226.111.
- Pesiridis G.S., Lee V.M., Trojanowski J.Q. Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis. Hum Mol Gen 2009; 18(R2): R156–R162, http://dx.doi.org/10.1093/hmg/ddp303.
- Highley J.R., Kirby J., Jansweijer J.A., Webb P.S., Hewamadduma C.A., Heath P.R., Higginbottom A., Raman R., Ferraiuolo L., Cooper-Knock J., McDermott C.J., Wharton S.B., Shaw P.J., Ince P.G. Loss of nuclear TDP-43 in amyotrophic lateral sclerosis (ALS) causes altered expression of splicing machinery and widespread dysregulation of RNA splicing in motor neurons. Neuropathol Appl Neurobiol 2014; 40(6): 670–685, http://dx.doi.org/10.1111/nan.12148.
- Miller J.W., Urbinati C.R., Teng-Umnuay P., Stenberg M.G., Byrne B.J., Thornton C.A., Swanson M.S. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J 2000; 19(17): 4439–4448, http://dx.doi.org/10.1093/emboj/19.17.4439.
- Osborne R.J., Lin X., Welle S., Sobczak K., O’Rourke J.R., Swanson M.S., Thornton C.A. Transcriptional and post-transcriptional impact of toxic RNA in myotonic dystrophy. Hum Mol Genet 2009; 18(8): 1471–1481, http://dx.doi.org/10.1093/hmg/ddp058.
- Nakamori M., Sobczak K., Puwanant A., Welle S., Eichinger K., Pandya S., Dekdebrun J., Heatwole C.R., McDermott M.P., Chen T., Cline M., Tawil R., Osborne R.J., Wheeler T.M., Swanson M.S., Moxley R.T. 3rd, Thornton C.A. Splicing biomarkers of disease severity in myotonic dystrophy. Ann Neurol 2013; 74(6): 862–872, http://dx.doi.org/10.1002/ana.23992.
- Miller D.T., Adam M.P., Aradhya S., Biesecker L.G., Brothman A.R., Carter N.P., Church D.M., Crolla J.A., Eichler E.E., Epstein C.J., Faucett W.A., Feuk L., Friedman J.M., Hamosh A., Jackson L., Kaminsky E.B., Kok K., Krantz I.D., Kuhn R.M., Lee C., Ostell J.M., Rosenberg C., Scherer S.W., Spinner N.B., Stavropoulos D.J., Tepperberg J.H., Thorland E.C., Vermeesch J.R., Waggoner D.J., Watson M.S., Martin C.L., Ledbetter D.H. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet 2010; 86(5): 749–764, http://dx.doi.org/10.1016/j.ajhg.2010.04.006.
- Chong W.W., Lo I.F., Lam S.T., Wang C.C., Luk H.M., Leung T.Y., Choy K.W. Performance of chromosomal microarray for patients with intellectual disabilities/developmental delay, autism, and multiple congenital anomalies in a Chinese cohort. Mol Cytogenet 2014; 7: 34, http://dx.doi.org/10.1186/1755-8166-7-34.
- Emy Dorfman L., Leite J.C., Giugliani R., Riegel M. Microarray-based comparative genomic hybridization analysis in neonates with congenital anomalies: detection of chromosomal imbalances. J Pediatr (Rio J) 2015; 91(1): 59–67, http://dx.doi.org/10.1016/j.jped.2014.05.007.
- Yuen R.K., Merkoulovitch A., MacDonald J.R., Vlasschaert M., Lo K., Grober E., Marshall C.R., Jarvi K.A., Kolomietz E., Scherer S.W. Development of a high-resolution Y-chromosome microarray for improved male infertility diagnosis. Fertil Steril 2014; 101(4): 1079–1085, http://dx.doi.org/10.1016/j.fertnstert.2013.12.027.
- Krepischi A.C., Capelli L.P., Silva A.G., de Araújo É.S., Pearson P.L., Heck B., da Costa C.M., de Camargo B., Rosenberg C. Large germline copy number variations as predisposing factor in childhood neoplasms. Future Oncol 2014; 10(9): 1627–1633, http://dx.doi.org/10.2217/fon.14.41.
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126(4): 663–676, http://dx.doi.org/10.1016/j.cell.2006.07.024.
- Woltjen K., Michael I.P., Mohseni P., Desai R., Mileikovsky M., Hämäläinen R., Cowling R., Wang W., Liu P., Gertsenstein M., Kaji K., Sung H.K., Nagy A. рiggyBack transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009; 458(7239): 766–770, http://dx.doi.org/10.1038/nature07863.
- Lowry W.E., Plath K. The many ways to make an iPS cell. Nat Biotechnol 2008; 26(11): 1246–1248, http://dx.doi.org/10.1038/nbt1108-1246.
- Fathi A., Hatami M., Hajihosseini V., Fattahi F., Kiani S., Baharvand H., Salekdeh G.H. Comprehensive gene expression analysis of human embryonic stem cells during differentiation into neural cells. PLoS One 2011; 6(7): e22856, http://dx.doi.org/10.1371/journal.pone.0022856.
- Dixon J.R., Jung I., Selvaraj S., Shen Y., Antosiewicz-Bourget J.E., Lee A.Y., Ye Z., Kim A., Rajagopal N., Xie W., Diao Y., Liang J., Zhao H., Lobanenkov V.V., Ecker J.R., Thomson J.A., Ren B. Chromatin architecture reorganization during stem cell differentiation. Nature 2015; 518(7539): 331–336, http://dx.doi.org/10.1038/nature14222.
- Lee J.H., Lee J.B., Shapovalova Z., Fiebig-Comyn A., Mitchell R.R., Laronde S., Szabo E., Benoit Y.D., Bhatia M. Somatic transcriptome priming gates lineage-specific differentiation potential of human-induced pluripotent stem cell states. Nat Commun 2014; 5: 5605, http://dx.doi.org/10.1038/ncomms6605.
- Busskamp V., Lewis N.E., Guye P., Ng A.H., Shipman S.L., Byrne S.M., Sanjana N.E., Murn J., Li Y., Li S., Stadler M., Weiss R., Church G.M. Rapid neurogenesis through transcriptional activation in human stem cells. Mol Syst Biol 2014; 10: 760, http://dx.doi.org/10.15252/msb.20145508.
- Dorn I., Klich K., Arauzo-Bravo M.J., Radstaak M., Santourlidis S., Ghanjati F., Radke T.F., Psathaki O.E., Hargus G., Kramer J., Einhaus M., Kim J.B., Kögler G., Wernet P., Schöler H.R., Schlenke P., Zaehres H. Erythroid differentiation of human induced pluripotent stem cells is independent of donor cell type of origin. Haematologica 2015; 100(1): 32–41, http://dx.doi.org/10.3324/haematol.2014.108068.
- Gabut M., Samavarchi-Tehrani P., Wang X., Slobodeniuc V., O’Hanlon D., Sung H.K., Alvarez M., Talukder S., Pan Q., Mazzoni E.O., Nedelec S., Wichterle H., Woltjen K., Hughes T.R., Zandstra P.W., Nagy A., Wrana J.L., Blencowe B.J. An alternative splicing switch regulates embryonic stem cell pluripotency and reprogramming. Cell 2011; 147(1): 132–146, http://dx.doi.org/10.1016/j.cell.2011.08.023.
- Rao S., Zhen S., Roumiantsev S., McDonald L.T., Yuan G.C., Orkin S.H. Differential roles of Sall4 isoforms in embryonic stem cell pluripotency. Mol Cell Biol 2010; 30(22): 5364–5380, http://dx.doi.org/10.1128/MCB.00419-10.
- Han H., Irimia M., Ross P.J., Sung H.K., Alipanahi B., David L., Golipour A., Gabut M., Michael I.P., Nachman E.N., Wang E., Trcka D., Thompson T., O’Hanlon D., Slobodeniuc V., Barbosa-Morais N.L., Burge C.B., Moffat J., Frey B.J., Nagy A., Ellis J., Wrana J.L., Blencowe B.J. MBNL proteins repress ES-cell-specific alternative splicing and reprogramming. Nature 2013; 498(7453): 241–245, http://dx.doi.org/10.1038/nature12270.
- Wu J.Q., Habegger L., Noisa P., Szekely A., Qiu C., Hutchison S., Raha D., Egholm M., Lin H., Weissman S., Cui W., Gerstein M., Snyder M. Dynamic transcriptomes during neural differentiation of human embryonic stem cells revealed by short, long, and paired-end sequencing. Proc Natl Acad Sci USA 2010; 107(11): 5254–5259, http://dx.doi.org/10.1073/pnas.0914114107.
- Salomonis N., Schlieve C.R., Pereira L., Wahlquist C., Colas A., Zambon A.C., Vranizan K., Spindler M.J., Pico A.R., Cline M.S., Clark T.A., Williams A., Blume J.E., Samal E., Mercola M., Merrill B.J., Conklin B.R. Alternative splicing regulates mouse embryonic stem cell pluripotency and differentiation. Proc Natl Acad Sci USA 2010; 107(23): 10514–10519, http://dx.doi.org/10.1073/pnas.0912260107.
- Royo S., Sainz B. Jr., Hernández-Jiménez E., Reyburn H., López-Collazo E., Guerra S. Differential induction of apoptosis, interferon signaling, and phagocytosis in macrophages infected with a panel of attenuated and nonattenuated poxviruses. J Virol 2014; 88(10): 5511–5523, http://dx.doi.org/10.1128/JVI.00468-14.
- Iglesias M.J., Reilly S.J., Emanuelsson O., Sennblad B., Pirmoradian Najafabadi M., Folkersen L., Mälarstig A., Lagergren J., Eriksson P., Hamsten A., Odeberg J. Combined chromatin and expression analysis reveals specific regulatory mechanisms within cytokine genes in the macrophage early immune response. PLoS One 7(2): e32306, http://dx.doi.org/10.1371/journal.pone.0032306.
- Saeed S., Quintin J., Kerstens H.H., Rao N.A., Aghajanirefah A., Matarese F., Cheng S.C., Ratter J., Berentsen K., van der Ent M.A., Sharifi N., Janssen-Megens E.M., Ter Huurne M., Mandoli A., van Schaik T., Ng A., Burden F., Downes K., Frontini M., Kumar V., Giamarellos-Bourboulis E.J., Ouwehand W.H., van der Meer J.W., Joosten L.A., Wijmenga C., Martens J.H., Xavier R.J., Logie C., Netea M.G., Stunnenberg H.G. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014; 345(6204): 1251086, http://dx.doi.org/10.1126/science.1251086.
- Olex A.L., Hiltbold E.M., Leng X., Fetrow J.S. Dynamics of dendritic cell maturation are identified through a novel filtering strategy applied to biological time-course microarray replicates. BMC Immunology 2010; 11: 41, http://dx.doi.org/10.1186/1471-2172-11-41.
- Wang P., Xue Y., Han Y., Lin L., Wu C., Xu S., Jiang Z., Xu J., Liu Q., Cao X. The STAT3-binding long noncoding RNA Inc-DC controls human dendritic cell differentiation. Science 2014; 344(6181): 310–313, http://dx.doi.org/10.1126/science.1251456.
- Zak D.E., Andersen-Nissen E., Peterson E.R., Sato A., Hamilton M.K., Borgerding J., Krishnamurty A.T., Chang J.T., Adams D.J., Hensley T.R., Salter A.I., Morgan C.A., Duerr A.C., De Rosa S.C., Aderem A., McElrath M.J. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci USA 2012; 109(50): E3503–E3512, http://dx.doi.org/10.1073/pnas.1208972109.
- Gattinoni L., Lugli E., Ji Y., Pos Z., Paulos C.M., Quigley M.F., Almeida J.R., Gostick E., Yu Z., Carpenito C., Wang E., Douek D.C., Price D.A., June C.H., Marincola F.M., Roederer M., Restifo N.P. A human memory T cell subset with stem cell-like properties. Nat Med 2011; 17(10): 1290–1298, http://dx.doi.org/10.1038/nm.2446.
- Kolokoltsova O.A., Yun N.E., Paessler S. Reactive astrogliosis in response to hemorrhagic fever virus: microarray profile of Junin virus-infected human astrocytes. Virol J 2014; 11: 126, http://dx.doi.org/10.1186/1743-422X-11-126.
- D’Aiuto L., Prasad K.M., Upton C.H., Viggiano L., Milosevic J., Raimondi G., McClain L., Chowdari K., Tischfield J., Sheldon M., Moore J.C., Yolken R.H., Kinchington P.R., Nimgaonkar V.L. Persistent Infection by HSV-1 is associated with changes in functional architecture of iPSC-derived neurons and brain activation patterns underlying working memory performance. Schizophr Bull 2015; 41(1): 123–132, http://dx.doi.org/10.1093/schbul/sbu032.
- Thomas E., Gonzalez V.D., Li Q., Modi A.A., Chen W., Noureddin M., Rotman Y., Liang T.J. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology 2012; 142(4): 978–988, http://dx.doi.org/10.1053/j.gastro.2011.12.055.
- Zamanian J.L., Xu L., Foo L.C., Nouri N., Zhou L., Giffard R.G., Barres B.A. Genomic analysis of reactive astrogliosis. J Neuroscience 2012; 32(18): 6391–6410, http://dx.doi.org/10.1523/JNEUROSCI.6221-11.2012.
- Shulzhenko N., Morgun A., Hsiao W., Battle M., Yao M., Gavrilova O., Orandle M., Mayer L., Macpherson A.J., McCoy K.D., Fraser-Liggett C., Matzinger P. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med 2011; 17(12): 1585–1594, http://dx.doi.org/10.1038/nm.2505.
- Rodrigues R., Grosso A.R., Moita L. Genome-wide analysis of alternative splicing during dendritic cell response to a bacterial challenge. PLoS One 2013; 8(4): e61975, http://dx.doi.org/10.1371/journal.pone.0061975.
- Ip J.Y., Tong A., Pan Q., Topp J.D., Blencowe B.J., Lynch K.W. Global analysis of alternative splicing during T-cell activation. RNA 2007; 13(4): 563–572, http://dx.doi.org/10.1261/rna.457207.










