Роль внеклеточного матрикса в синаптической пластичности при мозговых повреждениях
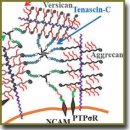
Внеклеточный матрикс мозга синтезируется нейронами и глиальными клетками и представляет собой молекулярную сеть, состоящую из полисахаридов и белков, которая заполняет пространство между клетками. Быстрое развитие внеклеточного матрикса в местах повреждений приводит к снижению регенеративной способности, в то время как разрушение внеклеточного матрикса — к ее увеличению. Изучение внеклеточного матрикса мозга, который может представлять собой потенциальную мишень для терапевтических воздействий, — важное направление современных исследований в области физиологии и медицины. В обзоре подробно рассмотрена структура внеклеточного матрикса мозга и его локализация в мозге, а также влияние матрикса на синаптическую передачу, обучение и память. Показана роль внеклеточного матрикса в восстановлении нейрональных связей после повреждений мозга, описано положительное воздействие на него фермента, разрушающего хондроитиназу АВС (хондроитин сульфат протеогликаны матрикса). Рассмотрены возможные влияния побочных эффектов разрушения хондроитин сульфат протеогликанов, используемых для восстановления нейрональных связей, на синаптическую передачу и память. Обозначены перспективы исследования роли внеклеточного матрикса мозга в норме и при патологии для дальнейшего развития науки.
- Nicholson C., Kamali-Zare P., Tao L. Brain extracellular space as a diffusion barrier. Comput Vis Sci 2011; 14(7): 309–325, https://doi.org/10.1007/s00791-012-0185-9.
- Dityatev A. Remodeling of extracellular matrix and epileptogenesis. Epilepsia 2010; 51: 61–65, https://doi.org/10.1111/j.1528-1167.2010.02612.x.
- Dityatev A., Schachner M. The extracellular matrix and synapses. Cell Tissue Res 2006; 326(2): 647–654, https://doi.org/10.1007/s00441-006-0217-1.
- Dityatev A., Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 2003; 4(6): 456–468, https://doi.org/10.1038/nrn1115.
- Lau L.W., Cua R., Keough M.B., Haylock-Jacobs S., Yong V.W. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci 2013; 14(10): 722–729, https://doi.org/10.1038/nrn3550.
- Bonneh-Barkay D., Wiley C.A. Brain Extracellular matrix in neurodegeneration. Brain Pathol 2009; 19(4): 573–585, https://doi.org/10.1111/j.1750-3639.2008.00195.x.
- Ruoslahti E. Brain extracellular matrix. Glycobiology 1996; 6(5): 489–492, https://doi.org/10.1093/glycob/6.5.489.
- Werner C., Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth 2007; 99(1): 4–9, https://doi.org/10.1093/bja/aem131.
- Kwok J.C.F., Dick G., Wang D., Fawcett J.W. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 2011; 71(11): 1073–1089, https://doi.org/10.1002/dneu.20974.
- Baeten K.M., Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 2011; 71(11): 1018–1039, https://doi.org/10.1002/dneu.20954.
- Rauch U. Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci 2004; 61(16), https://doi.org/10.1007/s00018-004-4043-x.
- Dityatev A., Rusakov D.A. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol 2011; 21(2): 353–359, https://doi.org/10.1016/j.conb.2010.12.006.
- Wright J.W., Harding J.W. Contributions of matrix metalloproteinases to neural plasticity, habituation, associative learning and drug addiction. Neural Plast 2009; 2009: 1–12, https://doi.org/10.1155/2009/579382.
- Michaluk P., Wawrzyniak M., Alot P., Szczot M., Wyrembek P., Mercik K., Medvedev N., Wilczek E., De Roo M., Zuschratter W., Muller D., Wilczynski G.M., Mozrzymas J.W., Stewart M.G., Kaczmarek L., Wlodarczyk J. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J Cell Sci 2011; 124(19): 3369–3380, https://doi.org/10.12 42/jcs.090852.
- Nagy V. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci 2006; 26(7): 1923–1934, https://doi.org/10.1523/jneurosci.4359-05.2006.
- Koprivica V. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 2005; 310(5745): 106–110, https://doi.org/10.1126/science.1115462.
- Lin R., Rosahl T.W., Whiting P.J., Fawcett J.W., Kwok J.C.F. 6-sulphated chondroitins have a positive influence on axonal regeneration. PLoS one 2011; 6(7): e21499, https://doi.org/10.1371/journal.pone.0021499.
- Shen Y., Tenney A.P., Busch S.A., Horn K.P., Cuascut F.X., Liu K., He Z., Silver J., Flanagan J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009; 326(5952): 592–596, https://doi.org/10.1126/science.1178310.
- Tauchi R., Imagama S., Natori T., Ohgomori T., Muramoto A., Shinjo R., Matsuyama Y., Ishiguro N., Kadomatsu K. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation 2012; 9(1): 53, https://doi.org/10.1186/1742-2094-9-53.
- García-Alías G., Lin R., Akrimi S.F., Story D., Bradbury E.J., Fawcett J.W. Therapeutic time window for the application of chondroitinase ABC after spinal cord injury. Exp Neurol 2008; 210(2): 331–338, https://doi.org/10.1016/j.expneurol.2007.11.002.
- Bradbury E.J., Moon L.D., Popat R.J., King V.R., Bennett G.S., Patel P.N., Fawcett J.W., McMahon S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002; 416(6881): 636–640, https://doi.org/10.1038/416636a.
- Dityatev A., Fellin T. Extracellular matrix in plasticity and epileptogenesis. Neuron Glia Biol 2008; 4(03): 235, https://doi.org/10.1017/s1740925x09000118.
- Pitkänen A., Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav 2009; 14(1): 16–25, https://doi.org/10.1016/j.yebeh.2008.09.023.
- Brenneke F., Schachner M., Elger C.E., Lie A.A. Up-regulation of the extracellular matrix glycoprotein tenascin-R during axonal reorganization and astrogliosis in the adult rat hippocampus. Epilepsy Res 2004; 58(2-3): 133–143, https://doi.org/10.1016/j.eplepsyres.2004.01.005.
- McGraw J., Hiebert G.W., Steeves J.D. Modulating astrogliosis after neurotrauma. J Neurosci Res 2001; 63(2): 109–115, https://doi.org/10.1002/1097-4547(20010115)63:2109::aid-jnr10023.0.co;2-j.
- Fitch M.T., Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 2008; 209(2): 294–301, https://doi.org/10.1016/j.expneurol.2007.05.014.
- Vargová L., Syková E. Extracellular space diffusion and extrasynaptic transmission. Physiol Res 2008; 57(Suppl 3): S89–S99.
- Stylianopoulos T., Poh M.-Z., Insin N., Bawendi M.G., Fukumura D., Munn L.L., Jain R.K. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J 2010; 99(5): 1342–1349, https://doi.org/10.1016/j.bpj.2010.06.016.
- Wlodarczyk J., Mukhina I., Kaczmarek L., Dityatev A. Extracellular matrix molecules, their receptors, and secreted proteases in synaptic plasticity. Dev Neurobiol 2011; 71(11): 1040–1053, https://doi.org/10.1002/dneu.20958.
- Frischknecht R., Heine M., Perrais D., Seidenbecher C.I., Choquet D., Gundelfinger E.D. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 2009; 12(7): 897–904, https://doi.org/10.1038/nn.2338.
- Hrabětová S., Masri D., Tao L., Xiao F., Nicholson C. Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J Physiol 2009; 587(16): 4029–4049, https://doi.org/10.1113/jphysiol.2009.170092.
- Sykova E., Nicholson C. Diffusion in brain extracellular space. physiological reviews. Physiol Rev 2008; 88(4): 1277–1340, https://doi.org/10.1152/physrev.00027.2007.
- Qian N., Sejnowski T.J. An electro-diffusion model for computing membrane potentials and ionic concentrations in branching dendrites, spines and axons. Biol Cybern 1989; 62(1): 1–15, https://doi.org/10.1007/bf00217656.
- Lopreore C.L., Bartol T.M., Coggan J.S., Keller D.X., Sosinsky G.E., Ellisman M.H., Sejnowski T.J. Computational modeling of three-dimensional electrodiffusion in biological systems: application to the node of ranvier. Biophys J 2008; 95(6): 2624–2635, https://doi.org/10.1529/biophysj.108.132167.
- Prydz K., Dalen K.T. Synthesis and sorting of proteoglycans. J Cell Sci 2000; 113(Pt 2): 193–205.
- Mecham R.P. Overview of extracellular matrix. Curr Protoc Cell Biol 2012; https://doi.org/10.1002/0471143030.cb1001s57.
- Karetko M., Skangiel-Kramska J. Diverse functions of perineuronal nets. Acta Neurobiol Exp (Wars) 2009; 69(4): 564–577.
- Celio M.R., Blümcke I. Perineuronal nets — a specialized form of extracellular matrix in the adult nervous system. Brain Res Brain Res Rev 1994; 19(1): 128–145, https://doi.org/10.1016/0165-0173(94)90006-x.
- Celio M.R., Spreafico R., De Biasi S., Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci 1998; 21(12): 510–515, https://doi.org/10.1016/s0166-2236(98)01298-3.
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol 1988; 4(1): 229–255, https://doi.org/10.1146/annurev.cb.04.110188.001305.
- Howell M.D., Gottschall P.E. Lectican proteoglycans, their cleaving metalloproteinases, and plasticity in the central nervous system extracellular microenvironment. Neuroscience 2012; 217: 6–18, https://doi.org/10.1016/j.neuroscience.2012.05.034.
- Grande-Allen K.J., Osman N., Ballinger M.L., Dadlani H., Marasco S., Little P.J. Glycosaminoglycan synthesis and structure as targets for the prevention of calcific aortic valve disease. Cardiovasc Res 2007; 76(1): 19–28, https://doi.org/10.1016/j.cardiores.2007.05.014.
- Rauch U. Brain matrix: structure, turnover and necessity. Biochem Soc Trans 2007; 35(4): 656–660, https://doi.org/10.1042/bst0350656.
- Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv Drug Deliv Rev 2016; 97: 4–27, https://doi.org/10.1016/j.addr.2015.11.001.
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci 2000; 57(2): 276–289, https://doi.org/10.1007/pl00000690.
- Fisher D., Xing B., Dill J., Li H., Hoang H.H., Zhao Z., Yang X.L., Bachoo R., Cannon S., Longo F.M., Sheng M., Silver J., Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci 2011; 31(40): 14051–1466, https://doi.org/10.1523/jneurosci.1737-11.2011.
- Bekku Y., Vargová L., Goto Y., Vorísek I., Dmytrenko L., Narasaki M., Ohtsuka A., Fässler R., Ninomiya Y., Syková E., Oohashi T. Bral1: its role in diffusion barrier formation and conduction velocity in the cnS. J Neurosci 2010; 30(8): 3113–3123, https://doi.org/10.1523/jneurosci.5598-09.2010.
- Anlar B., Gunel-Ozcan A. Tenascin-R: role in the central nervous system. Int J Biochem Cell Biol 2012; 44(9): 1385–1389, https://doi.org/10.1016/j.biocel.2012.05.009.
- Faissner A. The tenascin gene family in axon growth and guidance. Cell Tissue Res 1997; 290(2): 331–341, https://doi.org/10.1007/s004410050938.
- Pas J., Wyszko E., Rolle K., Rychlewski L., Nowak S., Zukiel R., Barciszewski J. Analysis of structure and function of tenascin-C. Int J Biochem Cell Biol 2006; 38(9): 1594–1602, https://doi.org/10.1016/j.biocel.2006.03.017.
- Bignami A., Hosley M., Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat Embryol (Berl) 1993; 188(5), https://doi.org/10.1007/bf00190136.
- Senkov O., Andjus P., Radenovic L., Soriano E., Dityatev A. Neural ECM molecules in synaptic plasticity, learning, and memory. Prog Brain Res 2014; 214: 53–80, https://doi.org/10.1016/b978-0-444-63486-3.00003-7.
- García-Alías G., Barkhuysen S., Buckle M., Fawcett J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci 2009; 12(9): 1145–1151, https://doi.org/10.1038/nn.2377.
- Kochlamazashvili G., Henneberger C., Bukalo O., Dvoretskova E., Senkov O., Lievens P.M., Westenbroek R., Engel A.K., Catterall W.A., Rusakov D.A., Schachner M., Dityatev A. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2+ channels. Neuron 2010; 67(1): 116–128, https://doi.org/10.1016/j.neuron.2010.05.030.
- Evers M.R., Salmen B., Bukalo O., Rollenhagen A., Bösl M.R., Morellini F., Bartsch U., Dityatev A., Schachner M. Impairment of L-type Ca2+ channel-dependent forms of hippocampal synaptic plasticity in mice deficient in the extracellular matrix glycoprotein tenascin-C. J Neurosci 2002; 22(16): 7177–7194.
- Gurevicius K., Kuang F., Stoenica L., Irintchev A., Gureviciene I., Dityatev A., Schachner M., Tanila H. Genetic ablation of tenascin-C expression leads to abnormal hippocampal CA1 structure and electrical activity in vivo. Hippocampus 2009; 19(12): 1232–1246, https://doi.org/10.1002/hipo.20585.
- Morellini F., Sivukhina E., Stoenica L., Oulianova E., Bukalo O., Jakovcevski I., Dityatev A., Irintchev A., Schachner M. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb Cortex 2010; 20(11): 2712–2727, https://doi.org/10.1093/cercor/bhq017.
- Bukalo O., Schachner M., Dityatev A. Hippocampal metaplasticity induced by deficiency in the extracellular matrix glycoprotein tenascin-R. J Neurosci 2007; 27(22): 6019–6028, https://doi.org/10.1523/jneurosci.1022-07.2007.
- Pujadas L., Gruart A., Bosch C., Delgado L., Teixeira C.M., Rossi D., de Lecea L., Martínez A., Delgado-García J.M., Soriano E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci 2010; 30(13): 4636–4649, https://doi.org/10.1523/jneurosci.5284-09.2010.
- Zhou X.H., Brakebusch C., Matthies H., Oohashi T., Hirsch E., Moser M., Krug M., Seidenbecher C.I., Boeckers T.M., Rauch U., Buettner R., Gundelfinger E.D., Fässler R. Neurocan is dispensable for brain development. Mol Cell Biol 2001; 21(17): 5970–5978, https://doi.org/10.1128/mcb.21.17.5970-5978.2001.
- Brakebusch C., Seidenbecher C.I., Asztely F., Rauch U., Matthies H., Meyer H., Krug M., Böckers T.M., Zhou X., Kreutz M.R., Montag D., Gundelfinger E.D., Fässler R. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol 2002; 22(21): 7417–7427, https://doi.org/10.1128/mcb.22.21.7417-7427.2002.
- Gogolla N., Caroni P., Luthi A., Herry C. Perineuronal nets protect fear memories from erasure. Science 2009; 325(5945): 1258–1261, https://doi.org/10.1126/science.1174146.
- Wang X.B., Bozdagi O., Nikitczuk J.S., Zhai Z.W., Zhou Q., Huntley G.W. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA 2008; 105(49): 19520–19525, https://doi.org/10.1073/pnas.0807248105.
- Maekawa M. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999; 285(5429): 895–898, https://doi.org/10.1126/science.285.5429.895.
- Amano M., Fukata Y., Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res 2000; 261(1): 44–51, https://doi.org/10.1006/excr.2000.5046.
- Murakoshi H., Wang H., Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 2011; 472(7341): 100–104, https://doi.org/10.1038/nature09823.
- LaPlaca M.C., Simon C.M., Prado G.R., Cullen D.K. CNS injury biomechanics and experimental models. Prog Brain Res 2007; 161: 13–26, https://doi.org/10.1016/s0079-6123(06)61002-9.
- Silver J., Miller J.H. Regeneration beyond the glial scar. Nature 2004; 5(2): 146–156, https://doi.org/10.1038/nrn1326.
- Anthony D.C., Couch Y. The systemic response to CNS injury. Exp Neurol 2014; 258: 105–111, https://doi.org/10.1016/j.expneurol.2014.03.013.
- Andersson P.-B., Perry V.H., Gordon S. The CNS acute inflammatory response to excitotoxic neuronal cell death. Immunol Lett 1991; 30(2): 177–181, https://doi.org/10.1016/0165-2478(91)90022-3.
- McDonald J.W., Sadowsky C. Spinal-cord injury. Lancet 2002; 359(9304): 417–425, https://doi.org/10.1016/s0140-6736(02)07603-1.
- Nakamura M., Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Research 2012; 23(1): 70–80, https://doi.org/10.1038/cr.2012.171.
- Soriano S.G., Piva S. Central nervous system inflammation. Eur J Anaesthesiol Suppl 2008; 25(Suppl 42): 154–159, https://doi.org/10.1017/s0265021507003390.
- Allan S.M., Rothwell N.J. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci 2003; 358(1438): 1669–1677, https://doi.org/10.1098/rstb.2003.1358.
- Faulkner J.R. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004; 24(9): 2143–2155, https://doi.org/10.1523/jneurosci.3547-03.2004.
- Asher R.A., Morgenstern D.A., Moon L.D.F., Fawcett J.W. Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog Brain Res 2001; 611–619, https://doi.org/10.1016/s0079-6123(01)32106-4.
- Chernoff E.A.G., O’hara C.M., Bauerle D., Bowling M. Matrix metalloproteinase production in regenerating axolotl spinal cord. Wound Repair Regen 2000; 8(4): 282–291, https://doi.org/10.1046/j.1524-475x.2000.00282.x.
- Aloisi F. Immune function of microglia. Glia 2001; 36(2): 165–179, https://doi.org/10.1002/glia.1106.
- Chen Y., Swanson R.A. Astrocytes and brain injury. J Cereb Blood Flow Metab 2003; 23(2): 137–149, https://doi.org/10.1097/01.wcb.0000044631.80210.3c.
- Johansson C.B., Momma S., Clarke D.L., Risling M., Lendahl U., Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999; 96(1): 25–34, https://doi.org/10.1016/s0092-8674(00)80956-3.
- Fawcett J.W., Asher R. The glial scar and central nervous system repair. Brain Res Bull 1999; 49(6): 377–391, https://doi.org/10.1016/s0361-9230(99)00072-6.
- Budinich C.S., Chen H., Lowe D., Rosenberger J.G., Bernstock J.D., McCabe J.T. Mouse brain PSA-NCAM levels are altered by graded-controlled cortical impact injury. Neural Plasticity 2012, https://doi.org/10.1155/2012/378307.
- Murphy J.A., Nickerson P.E.B., Clarke D.B. Injury to retinal ganglion cell axons increases polysialylated neural cell adhesion molecule (PSA-NCAM) in the adult rodent superior colliculus. Brain Research 2007; 1163: 21–32, https://doi.org/10.1016/j.brainres.2007.05.069.
- Kleene R., Mzoughi M., Joshi G., Kalus I., Bormann U., Schulze C., Xiao M.F., Dityatev A., Schachner M. NCAM-induced neurite outgrowth depends on binding of calmodulin to ncam and on nuclear import of ncam and fak fragments. J Neurosci 2010; 30(32): 10784–10798, https://doi.org/10.1523/jneurosci.0297-10.2010.
- Asher R.A., Morgenstern D.A., Fidler P.S., Adcock K.H., Oohira A., Braistead J.E., Levine J.M., Margolis R.U., Rogers J.H., Fawcett J.W. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci 2000; 20(7): 2427–2438.
- Lorber B., Hendriks W.J., Van der Zee C.E., Berry M., Logan A. Effects of LAR and PTP-BL phosphatase deficiency on adult mouse retinal cells activated by lens injury. Eur J Neurosci 2005; 21(9): 2375–2383, https://doi.org/10.1111/j.1460-9568.2005.04065.x.
- Pendleton J.C., Shamblott M.J., Gary D.S., Belegu V., Hurtado A., Malone M.L., McDonald J.W. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp Neurol 2013; 247: 113–121, https://doi.org/10.1016/j.expneurol.2013.04.003.
- Karus M., Ulc A., Ehrlich M., Czopka T., Hennen E., Fischer J., Mizhorova M., Qamar N., Brüstle O., Faissner A. Regulation of oligodendrocyte precursor maintenance by chondroitin sulphate glycosaminoglycans. Glia 2016; 64(2): 270–286, https://doi.org/10.1002/glia.22928.
- Siebert J.R., Osterhout D.J. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem 2011; 119(1): 176–188, https://doi.org/10.1111/j.1471-4159.2011.07370.x.
- Caggiano A.O., Zimber M.P., Ganguly A., Blight A.R., Gruskin E.A. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma 2005; 22(2): 226–39, https://doi.org/10.1089/neu.2005.22.226.
- Harris N.G., Mironova Y.A., Hovda D.A., Sutton R.L. Chondroitinase ABC enhances pericontusion axonal sprouting but does not confer robust improvements in behavioral recovery. J Neurotrauma 2010; 27(11): 1971–1982, https://doi.org/10.1089/neu.2010.1470.
- Roll L., Faissner A. Influence of the extracellular matrix on endogenous and transplanted stem cells after brain damage. Front Cell Neurosci 2014; 8, https://doi.org/10.3389/fncel.2014.00219.
- Schipper H.M. Astrocytes, brain aging, and neurodegeneration. Neurobiol Aging 1996; 17(3): 467–480, https://doi.org/10.1016/0197-4580(96)00014-0.
- Graham J.B., Neubauer D., Xue Q.-S., Muir D. Chondroitinase applied to peripheral nerve repair averts retrograde axonal regeneration. Exp Neurol 2007; 203(1): 185–195, https://doi.org/10.1016/j.expneurol.2006.08.004.
- Galtrey C.M., Asher R.A., Nothias F., Fawcett J.W. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain 2006; 130(4): 926–939, https://doi.org/10.1093/brain/awl372.
- Soleman S., Filippov M.A., Dityatev A., Fawcett J.W. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 2013; 253: 194–213, https://doi.org/10.1016/j.neuroscience.2013.08.050.
- Hill J.J., Jin K., Mao X.O., Xie L., Greenberg D.A. Intracerebral chondroitinase ABC and heparan sulfate proteoglycan glypican improve outcome from chronic stroke in rats. Proc Natl Acad Sci USA 2012; 109(23): 9155–9160, https://doi.org/10.1073/pnas.1205697109.
- Karimi-Abdolrezaee S., Eftekharpour E., Wang J., Schut D., Fehlings M.G. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci 2010; 30(5): 1657–1676, https://doi.org/10.1523/jneurosci.3111-09.2010.
- Fu M., Zhu B., Sun X., Luo D. The role of induced pluripotent stem cell (IPs) in the transplantation of glaucoma. Med Glas (Zenica) 2014; 11(2): 289–294.
- Rolls A., Shechter R., Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci 2009; 10(3): 235–241, https://doi.org/10.1038/nrn2591.
- Takano T., Oberheim N., Cotrina M.L., Nedergaard M. Astrocytes and ischemic injury. Stroke 2009; 40(3, Suppl 1): S8–S12, https://doi.org/10.1161/strokeaha.108.533166.
- Panickar K.S., Norenberg M.D. Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia 2005; 50(4): 287–298, https://doi.org/10.1002/glia.20181.
- Shen L.H., Li Y., Gao Q., Savant-Bhonsale S., Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia 2008; 56(16): 1747–1754, https://doi.org/10.1002/glia.20722.
- Bukalo O., Schachner M., Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 2001; 104(2): 359–369, https://doi.org/10.1016/s0306-4522(01)00082-3.
- Дембицкая Ю.В. Регуляция синаптической передачи активацией постсинаптических рецепторов, астроглией и внеклеточным матриксом мозга. Автореф. дис. … канд. биол. наук. Н. Новгород; 2015.
- Vedunova M., Sakharnova T., Mitroshina E., Perminova M., Pimashkin A., Zakharov Y., Dityatev A., Mukhina I. Seizure-like activity in hyaluronidase-treated dissociated hippocampal cultures. Front Cell Neurosci 2013; 7: 149, https://doi.org/10.3389/fncel.2013.00149.
- Mukhina I.V., Vedunova М.V., Sakharnova Т.А., Dityatev А.E. Modulation of network activity in dissociated hippocampal cultures by enzymatic digestion of extracellular matrix. Sovremennye tehnologii v medicine 2012; (1): 7–14.










