The Potential of Near-Infrared Spectroscopy as a Therapeutic Tool Following a Stroke (Review)
The advancement of novel technologies for the rehabilitation of post-stroke patients represents a significant challenge for a range of interdisciplinary fields. Near-infrared spectroscopy (NIRS) is an optical neuroimaging technique based on recording local hemodynamic changes at the cerebral cortex level. The technology is typically employed in post-stroke patients for diagnostic purposes, including the assessment of neuroplastic processes accompanying therapy, the study of hemispheric asymmetry, and the examination of functional brain networks. However, functional NIRS can also be used for therapeutic purposes, including the provision of biofeedback during rehabilitation tasks, as well as the navigation method during transcranial stimulation. The effectiveness of therapeutic NIRS application in stroke patients remains insufficiently studied, despite existing scientific evidence confirming its promising potential as a treatment method.
The review examines the published literature on the therapeutic applications of NIRS after stroke, evaluating its potential role in the rehabilitation process. The paper describes NIRS features, advantages, and disadvantages, determining its position among other neuroimaging technologies; analyzes the findings of neurophysiological studies, which justified the clinical trials of NIRS technology; and evaluates the results of the studies on the therapeutic use of NIRS in post-stroke patients. Two potential applications of NIRS for therapeutic purposes following a stroke were suggested: the first was to provide real-time feedback during movement training (motor or ideomotor ones, including that in brain–computer interface circuits), and the second was to facilitate navigation during transcranial stimulation.
Based on a comprehensive literature review, there were proposed and justified further research lines and development in this field.
Introduction
Stroke remains one of the major challenges of public health. According to the statistics, in 2019, stroke ranked third in the world among other causes of mortality and disability of population at a global scale. It is responsible for 5.7% (the range 5.1–6.2%) of the total life years lost due to different diseases. Stroke incidence dynamics is of greater concern. Over the period from 1990 to 2019, there was a considerable increase of primary cases of the disease — by 70.0% (variations 67.0–73.0%) [1]. In Russia in recent years there have been registered from 430,000 to 470,000 stroke cases annually; during a year following a stroke 95,500 people completely cease their work activities, and over the half of those who survived require assistance and care from others [2]. At every stage of medical rehabilitation, there is the lack of the staff to provide physical therapy (basic methods of therapeutic exercises) [3], therefore, the development of available rehabilitation technologies is still much-needed. Innovative technologies can compensate to different extents for the intensity of physical therapy insufficient for functional recovery and be used when there are some constraints in providing therapeutic exercises or accompany the therapy at various stages of medical rehabilitation [4–7].
Near-infrared spectroscopy (NIRS) is a noninvasive optical imaging technique that records the changes in the concentrations of certain hemoglobin fractions of microcirculation up to 3 cm deep from skin covering [8, 9]. Functional NIRS (fNIRS) similarly to functional magnetic resonance imaging (fMRI) enables to record the cerebral cortex activity by measuring the local oxygenation dynamics, and at the same time it being portable, resistant to electromagnetic interferences, and motion artifacts, and much cheaper technology [10–12].
Currently, fNIRS is widely used in clinical-based studies in post-stroke patients to assess neurophysiological characteristics of rehabilitation efficiency [13–17] and recovery prognosis [18, 19], to study recovery mechanisms [20, 21], hemispheric relation and asymmetry [21–25]. However, fNIRS can also be employed as a therapeutic technology for training with biological feedback (BFB), in brain-computer interface (BCI) and for functional navigation when using neuromodulation methods [9, 26, 27].
In contrast to diagnostic strategies of fNIRS use, its therapeutic ones are underexplored.
The present review aimed at analyzing the published data on the therapeutic application of fNIRS after stroke to determine a possible position of the technology in the rehabilitation process and ground further research lines and development in the sphere.
Literature source searching methodology
To search the review articles devoted to NIRS used after stroke and published within the recent 5 years in PubMed/MEDLINE the following enquiry was used: (near-infrared spectroscopy [tiab] OR NIRS [tiab]) AND (stroke [mh] OR stroke [tiab]) AND (meta-analysis [pt] OR review [pt] OR systematic review [pt]) AND 2020:2024 [dp]. The searching date is June 10, 2024.
To search literature sources concerning clinical research of using NIRS following a stroke in PubMed/MEDLINE the following enquiry was used: (near-infrared spectroscopy [tiab] OR NIRS [tiab]) AND (stroke [mh] OR stroke [tiab]) AND (clinical trial [pt] OR randomized controlled trial [pt]). The searching date is June 17, 2024.
Available Russian publications were searched in eLIBRARY.RU by key words “NIRS” and “near infrared spectroscopy”. The searching date is July 5, 2024.
Additionally, clinical trial protocols were searched at https://clinicaltrials.gov by key words “near-infrared spectroscopy” and “stroke”. The searching date is July 8, 2024.
Features, advantages, and disadvantages of functional near-infrared spectroscopy
NIRS technique enables to record the changes in hemoglobin concentration in the microcirculation (in the vessels with a diameter of less than 1 mm) at a depth of up to 3 cm from the head surface. For this purpose, it has been used in biomedical studies since the late 1970s [28]. It is based on the light ability in near-infrared range to penetrate biological tissues, where it is absorbed and mainly scattered. Radiation attenuation in the range 650–1000 nm is primarily due to its absorption by hemoglobin [28–30].
fNIRS systems utilize the light sources with an optic window from 650 to 1000 nm, light detectors, as well as flexible fiber optics. Moreover, fiber optics are suitable for any head position and do not require study subject immobilization. Sources and detectors are primarily fixed in an elastic cap at a distance of 1.5–5.0 cm (more frequently — 3 cm) from one another. A signal recording channel includes a pair: emitter–detector. Based on the information on incident and outgoing light and applying a modified Bouguer–Lambert–Beer law, light attenuation is calculated. This enables real-time measurement of oxygenated hemoglobin (HbO), deoxygenated hemoglobin (HbR), and total hemoglobin (HbT) concentrations [28, 29, 31]. Current NIRS systems can contain up to several hundreds of channels with time resolution up to 250 Hz and space resolution up to 10 mm [8, 28].
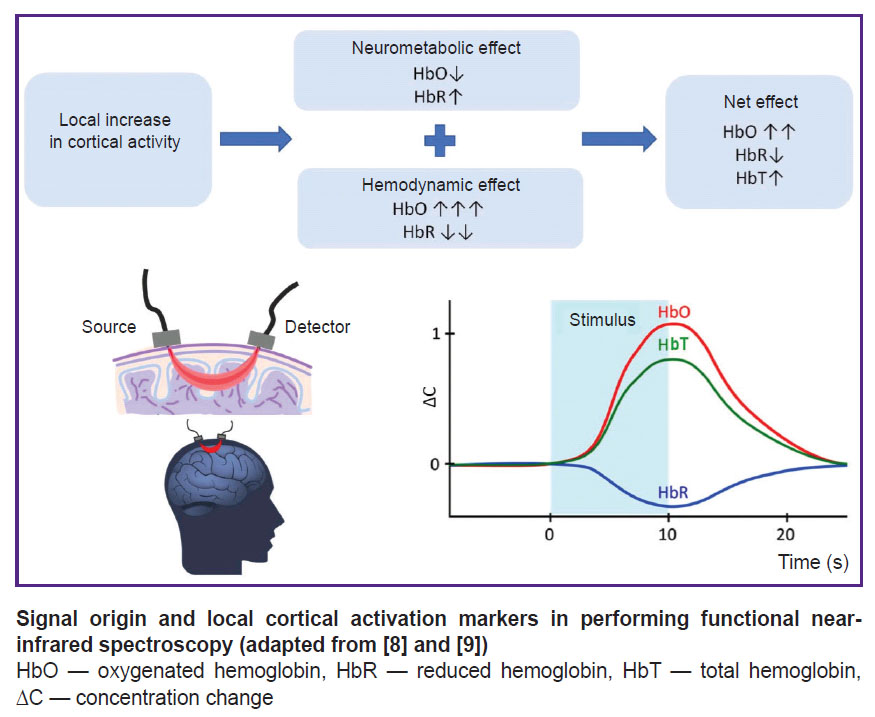
fNIRS performing is based on the following processes accompanying the brain cortex activation. The increase in cortical neuron activity results in two effects: 1) neurometabolic consisting in HbO concentration decrease and HbR concentration increase due to increased tissue breathing; 2) hemodynamic, which, in contrast, leads to HbO concentration increase and HbR concentration decrease due to local blood flow increase. Since a hemodynamic effect significantly outweighs the metabolic one, the markers of local cortical activation during fNIRS include the increase in HbO concentration and the decrease in HbR concentration. The increase in HbO concentration also results in HbT concentration growth (see the Figure) [8, 9].
As a neuroimaging tool, fNIRS offers the following advantages: ease of administration (requires neither electrode gel application nor the study subject immobilization); portability; high movement interference resistance; electromagnetic compatibility; enhanced signal reliability (enabled by simultaneous monitoring of multiple parameters: HbO, HbR, and HbT concentrations); relatively low cost [8, 28]. Due to its portability and resistance to motion artifacts, fNIRS — unlike fMRI — allows for the performance of functional tasks in seated or standing positions. Owing to the mentioned advantages, fNIRS can be employed in a number of situations where fMRI is impractical: in children [32–34], in people with metal implants, those suffering from cognitive disorders, claustrophobia, and other conditions [9, 35]. The feasibility of using NIRS in home settings after stroke has been demonstrated [36]. In applications where fNIRS can serve as an alternative to electroencephalography (EEG) or fMRI, for example, for BFB, the technique is a compromise solution between good time resolution of EEG and good space resolution of fMRI [9]. The main fNIRS disadvantages are the following: limited imaging depth (hemodynamic response can be assessed at a cortical level, but not in deep brain structures), relatively low spatial resolution, temporal signal delay caused by the slow nature of hemodynamic response, signal interference from the patient’s body temperature fluctuations and cardiac activity (e.g., heartbeat). Furthermore, current NIRS technologies are restricted to measuring relative changes in hemodynamic parameters rather than providing absolute quantitative values [8, 9, 27, 31].
Neurofeedback control by NIRS signal
NIRS can be used for feedback during motor imagery, i.e., the mental reconstruction of the sense of motion [37–41]. The motor imagery is accompanied by the activation of brain structures involved in the process of a real movement. It is supposed to have a positive impact on neuroplasticity, which underlies the motor recovery [42]. It is worth noting that motor imagery training (ideomotor training) is possible even in severe limb paresis, when active motor exercises are limited. A positive effect of ideomotor trainings on motor function recovery after stroke was demonstrated in many studies [43, 44]. Biofeedback enables to control a patient performing a mental task of the motor imagery improving the efficiency of such training.
An ideomotor training with feedback using NIRS involves the following stages. An operator (a patient or a healthy volunteer) takes an instruction to imagine certain limb movement; and the mental activity is accompanied by the changes in HbO and HbR concentrations in the cortical areas responsible for the movement, it being registered by NIRS; then NIRS signal is recognized by computer programs and converted into a signal for feedback. The feedback is delivered to the operator (in its easiest form — as a visual signal on a computer monitor) [37–41]. The most popular form of such approach implementation is the use of BCI technology [45–47].
According to the latest definition, BCI is a system that measures the brain activity and converts it (approximately) in real-time into functionally useful output signals for replacement, recovery, enhancement, supplement, and/or improvement of natural output brain signals, by that changing the current interaction processes between the brain and its external or internal environment [48]. EEG-based BCI is used for motor imagery trainings after stroke, and a signal of the brain activation, as a rule, is the desynchronization of the sensorimotor rhythm. Despite a number of meta-analyses have demonstrated EEG–BCI efficiency after stroke, the main issues of the technology implementation are the requirement to apply electrode gel to the patient’s scalp and its low resistance to interference [49].
The feasibility of NIRS–BCI application as an alternative to EEG–BCI to perform ideomotor trainings was shown in the early 2000s in healthy volunteers [37–39]. Later, some controlled studies with healthy volunteers stated real rather than fictitious feedback by NIRS signal to induce specific and focused activation of the motor cortex during movement imaging [50–52], and the course of such trainings was accompanied by improved agility of hand movement [52]. Moreover, repetitive trainings with NIRS feedback in healthy people were demonstrated to improve the ability to imagine movements both according to the assessment of the subjects themselves [50] and according to neuroimaging data (there was the increased specificity of motor cortex activation) [50, 51].
The healthy volunteers in the study [53] were offered to control the activity of the supplementary motor cortex with BFB using NIRS signal or a fictitious BFB. However, there were specified none certain strategies to control signals, e.g. motor imagery strategies. Finally, only a group with real feedback was observed to have increased activity of the supplementary motor cortex and improved postural balance but not the agility of hand movement.
The study [54] investigated a hybrid BCI based on EEG and NIRS recording during the movement imaging by healthy volunteers. The brain activity signals were converted into the control over functional electric stimulation (FES) of the hand muscles. In the control group, FES was triggered randomly, not by BCI signals. As a result of trainings, the BCI group, compared with the control one, had more expressed cortical activation according to EEG and NIRS parameters.
The findings of the studies with healthy volunteers provided justification of carrying out several clinical trials of using feedback by NIRS signal in post-stroke patients [55–60], two of the studies were randomized controlled trials (RCT) (see the Table).
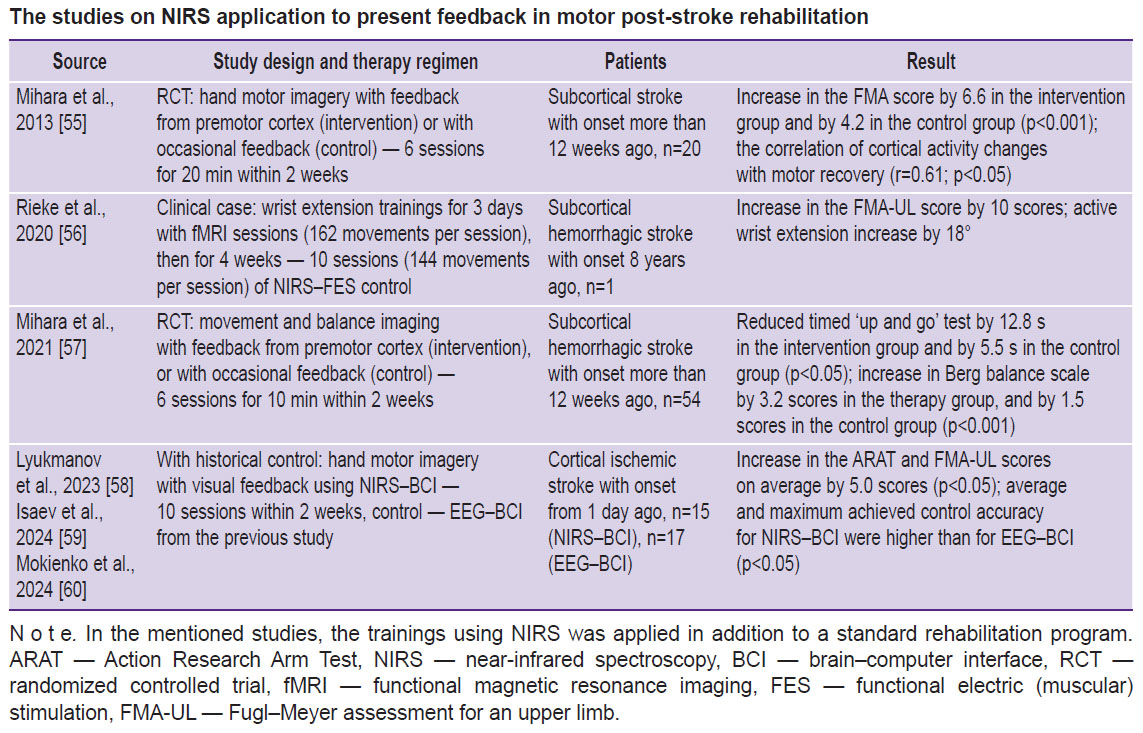 |
The studies on NIRS application to present feedback in motor post-stroke rehabilitation |
RCTs have demonstrated the advantage of ideomotor trainings with NIRS feedback for the recovery of hand movement [55], balance, and walking [57]. In both, studies the use of real but not fictitious feedback was accompanied by significant activation of motor associative cortex (premotor, supplementary motor).
The NIRS–BCI technology in a more complete implementation was used in the study [56], which describes the results of controlling functional electrical stimulation of muscles using brain NIRS signals in a clinical case. A patient first had three fMRI-feedback sessions to determine the cerebral cortex areas activated in wrist extension. It was taken into consideration when choosing NIRS channels location. During trainings with BCI, the patient attempted to straighten his wrist, the brain NIRS signals being converted into the control commands of wrist muscular FES to facilitate movements. Despite the neurophysiological ground of the approach and the impressive result of the hand motor recovery following 8 years after stroke, in this case, NIRS–BCI–FES technology contribution is unclear, since each training itself included more than 140 movement repetitions.
The studies [58–60] using NIRS–BCI involved the patients imagined the hand motor task from Action Research Arm Test (ARAT), which performing was the most difficult due to the post-stroke paresis. The results of the hand movement recovery and BCI control quality were higher when using NIRS–BCI compared to EEG–BCI. The authors explained it by the fact that the cortical activity indicators in NIRS are several parameters (HbO, HbR concentration measurement) that facilitates the task of the BCI classifier. The second explanation was the grater interference immunity of NIRS compared to EEG [60].
Navigation through near-infrared for neurostimulation
Rhythmic transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation method that modulates the excitability of the target cortical zone. According to current views, rTMS effect mechanisms are based on the induction of long-term potentiation or long-term depression [61]. TMS effects may result from either neuromodulation of the stimulated brain regions or changes in functional brain networks connecting several remote areas [62–64].
Numerous systematic reviews and meta-analyses described the efficiency of the approach in post-stroke patients in relation to recovery of motor function [63, 65], cognitive function [66, 67], speaking [68], swallowing [69], treatment of post-stroke central pain syndrome [70, 71], or post-stroke depression [72].
Despite widespread application of rTMS in post-stroke patients, the search for optimal parameters and the development of strategies to select individualized rTMS protocols remain ongoing [61, 64]. Until now the mechanisms of TMS effect on target cortex have been underexplored, and it is unclear how the local effect is spreading in the central nervous system. Moreover, the problem of choosing stimulation points has no solution so far. In stimulating motor areas, the predominant approach includes the location determination of the point with the highest amplitude of motor evoked potentials. However, this approach often does not work due to high individual excitability threshold, presence of stroke focal focus, or due to other unstudied reasons. Therefore, some alternative rTMS navigation methods are needed [62, 73].
Optically measured NIRS signals are not susceptible to electromagnetic interference, and the technology does not affect the magnetic properties of TMS coil that enables to use these approaches simultaneously. Conducting NIRS during rTMS enables to make an objective quantitative assessment of neurophysiological responses during stimulation in both spatial and time coordinates. This makes it possible, including in real-time mode, to adjust the parameters of rTMS and select stimulation targets [74, 75].
In the study [76], the patients with aphasia resulting from stroke with an onset of more than 12 months ago underwent the course of 10 sessions of therapeutic rTMS in addition to intensive speech therapy. Before the course of therapy using NIRS, the cortical activation areas were determined during the performance of a speech task. Based on NIRS findings, the rTMS lateralization and mode were determined. There was observed a pronounced therapeutic effect, however, the study involved no control group, and the sample included 8 patients only. Thus, the authors proposed a personalized approach for choosing rTMS protocol based on individual neuroimaging using NIRS.
The double-blind RCT involving patients with post-stroke arm paresis compared the protocols of rTMS navigation using NIRS and motor evoked potentials [77]. The points for rTMS were determined in all cases when NIRS was used as navigation, even when the motor evoked responses could not be recorded. After a 10-day therapy course, both groups, unlike the sham stimulation group, showed improvement in upper extremity motor function based on two assessment scales. However, improvement in elbow joint movements was observed only in the NIRS navigation group. These findings highlight the feasibility of combining rTMS and NIRS technologies to restore motor function after stroke.
Further lines of research and developments
Taking into consideration the importance of post-stroke rehabilitation, unique fNIRS characteristics, and the open scientific practical issues of using neuromodulation methods, supplementary research of the therapeutic application of the method remain in demand. To compare the clinical efficiency of NIRS–BCI with more studied, though less convenient EEG–BCI technology, it is necessary to carry out prospective comparative studies in parallel groups. Moreover, further research can also use NIRS–BCI with feedback through the exoskeleton of upper [78, 79] or lower [80] limbs. However, FES can become the most physiological and effective feedback in BCI [81–84].
Motor imagery trainings, in addition to motor recovery, can contribute to the improvement of cognitive functions and cognitive components of a motor process (e.g., motion planning) [85, 86]. Therefore, it is necessary to pay attention to studying the efficiency of NIRS–BCI trainings in relation to recovering cognitive functions in post-stroke patients.
One of the novel applications of feedback by NIRS signal can be the recovery of swallowing. Several studies involving healthy volunteers managed to achieve the successful regulation of motor cortex activity (inferior frontal gyrus) associated with swallowing using feedback through NIRS [87–89].
An important practical aspect is further cost-cutting of NIRS technology and its simplification for patient’s self-use. There have already been published several clinical trial protocols of NIRS-based developments to perform motor and mental trainings at home [90, 91].
Open public access to structured datasets of brain NIRS signal recordings will accelerate the improvement of algorithms for their processing and classification. By now, there have been published several NIRS data sets obtained from healthy people [92–95], and just one — from post-stroke patients [59].
The hybrid EEG–NIRS BCI is of great interest for brain-machine interface developers [54, 96–99]. The combination of two methods of signal recording will enable to improve BCI control quality, however, hybrid BCI appears to be less convenient for clinical practice.
In respect to any neurorehabilitation techniques, it is important to understand the underlying models of motor control and learning [100]. Currently, there remains the understudied issue — the activity of which cortical areas and in what succession is appropriate to modulate using feedback to achieve more pronounced functional recovery [9, 27]. A number of carried out studies showed biofeedback using NIRS to be performed by signals from associative motor areas, which may be involved in movement suppression. Therefore, supplementary investigations are needed to justify the selection of the brain signal sources. Moreover, it is highly interesting to set up the feedback based on the connectivity of a functional network of several areas rather than the signals from certain areas [9].
The results of two clinical studies of rTMS navigation using NIRS have been described, and their protocols have been published [101, 102]. However, despite the existing theoretical prerequisites for further clinical studies, this direction is developing rather slowly. The technical difficulties related to the application of hybrid NIRS–TMS systems (or NIRS in combination with transcranial electrical stimulation [103, 104]) should not prevent from carrying out research in this field, since they can improve understanding of fundamental aspects of motor recovery after stroke.
Conclusion
Thus, by now there have been suggested two trends of fNIRS application for therapy after stroke: to present feedback during movement trainings (motor or ideomotor, including those in BCI contour) and for navigation when performing transcranial stimulation.
Despite the long-term existence of NIRS technology, in post-stroke rehabilitation it has been primarily studied as a diagnostic rather than therapeutic tool.
Having a number of shortcomings, NIRS exhibits certain advantages over some alternative approaches of feedback presenting during movement trainings or rTMS navigation methods. Increasing technology availability, including through the emergence of new commercial products, as well as additional well-designed clinical trials, will better justify fNIRS place in post-stroke rehabilitation protocols.
Study funding. The work was carried out within the framework of the state task of the Ministry of Science and Higher Education of the Russian Federation, registration number — 1021062411635-8-3.1.4.
Conflict of interest. The author declares no conflict of interest related to the present study.
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021; 20(10): 795–820, https://doi.org/10.1016/S1474-4422(21)00252-0.
- Ignatyeva V.I., Voznyuk I.A., Shamalov N.A., Reznik A.V., Vinitskiy A.A., Derkach E.V. Social and economic burden of stroke in Russian Federation. Zhurnal nevrologii i psikhiatrii im. S.S. Korsakova 2023; 123(8–2): 5–15, https://doi.org/10.17116/jnevro20231230825.
- Builova T.V., Zverev Yu.P., Ivanova G.E., Kuzminova T.A. Current requirements for universities planning to train physical rehabilitation specialists in the context of the new medical rehabilitation model in the Russian Federation: a review. Vestnik vosstanovitel’noj mediciny 2022; 21(4): 17–26, https://doi.org/10.38025/2078-1962-2022-21-4-17-26.
- Hatem S.M., Saussez G., Della Faille M., Prist V., Zhang X., Dispa D., Bleyenheuft Y. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci 2016; 10: 442, https://doi.org/10.3389/fnhum.2016.00442.
- Petrikov S.S., Grechko A.V., Shchelkunova I.G., Zavaliy Ya.P., Khat’kova S.E., Zavaliy L.B. New perspectives of motor rehabilitation of patients after focal brain lesions. Voprosy neirokhirurgii im. N.N. Burdenko 2019; 83(6): 90–99, https://doi.org/10.17116/neiro20198306190.
- Ceradini M., Losanno E., Micera S., Bandini A., Orlandi S. Immersive VR for upper-extremity rehabilitation in patients with neurological disorders: a scoping review. J Neuroeng Rehabil 2024; 21(1): 75, https://doi.org/10.1186/s12984-024-01367-0.
- Saway B.F., Palmer C., Hughes C., Triano M., Suresh R.E., Gilmore J., George M., Kautz S.A., Rowland N.C. The evolution of neuromodulation for chronic stroke: from neuroplasticity mechanisms to brain-computer interfaces. Neurotherapeutics 2024; 21(3):e00337, https://doi.org/10.1016/j.neurot.2024.e00337.
- Soekadar S.R., Kohl S.H., Mihara M., von Lühmann A. Optical brain imaging and its application to neurofeedback. Neuroimage Clin 2021; 30: 102577, https://doi.org/10.1016/j.nicl.2021.102577.
- Huo C., Xu G., Xie H., Chen T., Shao G., Wang J., Li W., Wang D., Li Z. Functional near-infrared spectroscopy in non-invasive neuromodulation. Neural Regen Res 2024; 19(7): 1517–1522, https://doi.org/10.4103/1673-5374.387970.
- Yang M., Yang Z., Yuan T., Feng W., Wang P. A systemic review of functional near-infrared spectroscopy for stroke: current application and future directions. Front Neurol 2019; 10: 58, https://doi.org/10.3389/fneur.2019.00058.
- Cui X., Bray S., Bryant D.M., Glover G.H., Reiss A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 2011; 54(4): 2808–2821, https://doi.org/10.1016/j.neuroimage.2010.10.069.
- Xu G., Huo C., Yin J., Zhong Y., Sun G., Fan Y., Wang D., Li Z. Test-retest reliability of fNIRS in resting-state cortical activity and brain network assessment in stroke patients. Biomed Opt Express 2023; 14(8): 4217–4236, https://doi.org/10.1364/BOE.491610.
- Xie H., Li X., Xu G., Huo C., Fan Y., Li Z., Dou Z. Effects of transcranial magnetic stimulation on dynamic functional networks in stroke patients as assessed by functional near-infrared spectroscopy: a randomized controlled clinical trial. Cereb Cortex 2023; 33(24): 11668–11678, https://doi.org/10.1093/cercor/bhad404.
- Li Y., Yan Z.P., Zhang N.N., Ni J., Wang Z.Y. Investigation into the effectiveness of combining transcranial direct current stimulation and transcutaneous electrical nerve stimulation as treatment options for poststroke shoulder pain by utilizing functional near-infrared spectroscopy. Ther Clin Risk Manag 2023; 19: 875–887, https://doi.org/10.2147/TCRM.S431816.
- Yoo M., Chun M.H., Hong G.R., Lee C., Lee J.K., Lee A. Effects of training with a powered exoskeleton on cortical activity modulation in hemiparetic chronic stroke patients: a randomized controlled pilot trial. Arch Phys Med Rehabil 2023; 104(10): 1620–1629, https://doi.org/10.1016/j.apmr.2023.05.012.
- Wang Q., Dai W., Xu S., Zhu S., Sui Y., Kan C., Shen Y., Zhu Y., Guo C., Wang T. Brain activation of the PFC during dual-task walking in stroke patients: a systematic review and meta-analysis of functional near-infrared spectroscopy studies. Front Neurosci 2023; 17: 1111274, https://doi.org/10.3389/fnins.2023.1111274.
- Dai L., Zhang W., Zhang H., Fang L., Chen J., Li X., Yu H., Song J., Chen S., Zheng B., Zhang Y., Li Z. Effects of robot-assisted upper limb training combined with intermittent theta burst stimulation (iTBS) on cortical activation in stroke patients: a functional near-infrared spectroscopy study. NeuroRehabilitation 2024; 54(3): 421–434, https://doi.org/10.3233/NRE-230355.
- Ansado J., Chasen C., Bouchard S., Northoff G. How brain imaging provides predictive biomarkers for therapeutic success in the context of virtual reality cognitive training. Neurosci Biobehav Rev 2021; 120: 583–594, https://doi.org/10.1016/j.neubiorev.2020.05.018.
- Tamashiro H., Kinoshita S., Okamoto T., Urushidani N., Abo M. Effect of baseline brain activity on response to low-frequency rTMS/intensive occupational therapy in poststroke patients with upper limb hemiparesis: a near-infrared spectroscopy study. Int J Neurosci 2019; 129(4): 337–343, https://doi.org/10.1080/00207454.2018.1536053.
- Huo C., Xu G., Li Z., Lv Z., Liu Q., Li W., Ma H., Wang D., Fan Y. Limb linkage rehabilitation training-related changes in cortical activation and effective connectivity after stroke: a functional near-infrared spectroscopy study. Sci Rep 2019; 9(1): 6226, https://doi.org/10.1038/s41598-019-42674-0.
- Delorme M., Vergotte G., Perrey S., Froger J., Laffont I. Time course of sensorimotor cortex reorganization during upper extremity task accompanying motor recovery early after stroke: an fNIRS study. Restor Neurol Neurosci 2019; 37(3): 207–218, https://doi.org/10.3233/RNN-180877.
- Chen S., Zhang X., Chen X., Zhou Z., Cong W., Chong K., Xu Q., Wu J., Li Z., Lin W., Shan C. The assessment of interhemispheric imbalance using functional near-infrared spectroscopic and transcranial magnetic stimulation for predicting motor outcome after stroke. Front Neurosci 2023; 17: 1231693, https://doi.org/10.3389/fnins.2023.1231693.
- Huo C., Sun Z., Xu G., Li X., Xie H., Song Y., Li Z., Wang Y. fNIRS-based brain functional response to robot-assisted training for upper-limb in stroke patients with hemiplegia. Front Aging Neurosci 2022; 14: 1060734, https://doi.org/10.3389/fnagi.2022.1060734.
- Ni J., Jiang W., Gong X., Fan Y., Qiu H., Dou J., Zhang J., Wang H., Li C., Su M. Effect of rTMS intervention on upper limb motor function after stroke: a study based on fNIRS. Front Aging Neurosci 2023; 14: 1077218, https://doi.org/10.3389/fnagi.2022.1077218.
- He X., Lei L., Yu G., Lin X., Sun Q., Chen S. Asymmetric cortical activation in healthy and hemiplegic individuals during walking: a functional near-infrared spectroscopy neuroimaging study. Front Neurol 2023; 13: 1044982, https://doi.org/10.3389/fneur.2022.1044982.
- Kohl S.H., Mehler D.M.A., Lührs M., Thibault R.T., Konrad K., Sorger B. The potential of functional near-infrared spectroscopy-based neurofeedback — a systematic review and recommendations for best practice. Front Neurosci 2020; 14: 594, https://doi.org/10.3389/fnins.2020.00594.
- Zhao Y.N., Han P.P., Zhang X.Y., Bi X. Applications of functional near-infrared spectroscopy (fNIRS) neuroimaging during rehabilitation following stroke: a review. Med Sci Monit 2024; 30: e943785, https://doi.org/10.12659/MSM.943785.
- Ferrari M., Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012; 63(2): 921–935, https://doi.org/10.1016/j.neuroimage.2012.03.049.
- Tarasov A.P., Egorov A.I., Drozdov D.V. Optical tissue oximetry: problems of application in functional diagnostics. Meditsinskiy alfavit 2017; 2(22): 48–52.
- Isaev M.R., Oganesyan V.V., Husek D., Snasel V. Modeling light propagation through the tissues of the head taking account of scattering anisotropy to optimize the positioning of irradiation detectors and sources in a brain-computer interface based on near infrared spectroscopy. Neurosci Behav Physi 2018; 48: 1158–1163, https://doi.org/10.1007/s11055-018-0680-7.
- Chen W.L., Wagner J., Heugel N., Sugar J., Lee Y.W., Conant L., Malloy M., Heffernan J., Quirk B., Zinos A., Beardsley S.A., Prost R., Whelan H.T. Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: advances and future directions. Front Neurosci 2020; 14: 724, https://doi.org/10.3389/fnins.2020.00724.
- Harrar D.B., Sun L.R., Segal J.B., Lee S., Sansevere A.J. Neuromonitoring in children with cerebrovascular disorders. Neurocrit Care 2023; 38(2): 486–503, https://doi.org/10.1007/s12028-023-01689-2.
- Felling R.J., Kamerkar A., Friedman M.L., Said A.S., LaRovere K.L., Bell M.J., Bembea M.M. Neuromonitoring during ECMO support in children. Neurocrit Care 2023; 39(3): 701–713, https://doi.org/10.1007/s12028-023-01675-8.
- Guo Y., Li Y., Liu F., Lin H., Sun Y., Zhang J., Hong Q., Yao M., Chi X. Association between neural prosody discrimination and language abilities in toddlers: a functional near-infrared spectroscopy study. BMC Pediatr 2024; 24(1): 449, https://doi.org/10.1186/s12887-024-04889-7.
- Ishii-Takahashi A., Takizawa R., Nishimura Y., Kawakubo Y., Kuwabara H., Matsubayashi J., Hamada K., Okuhata S., Yahata N., Igarashi T., Kawasaki S., Yamasue H., Kato N., Kasai K., Kano Y. Prefrontal activation during inhibitory control measured by near-infrared spectroscopy for differentiating between autism spectrum disorders and attention deficit hyperactivity disorder in adults. Neuroimage Clin 2013; 4: 53–63, https://doi.org/10.1016/j.nicl.2013.10.002.
- Lee Friesen C., Lawrence M., Ingram T.G.J., Boe S.G. Home-based portable fNIRS-derived cortical laterality correlates with impairment and function in chronic stroke. Front Hum Neurosci 2022; 16: 1023246, https://doi.org/10.3389/fnhum.2022.1023246.
- Coyle S., Ward T., Markham C., McDarby G. On the suitability of near-infrared (NIR) systems for next-generation brain-computer interfaces. Physiol Meas 2004; 25(4): 815–822, https://doi.org/10.1088/0967-3334/25/4/003.
- Sitaram R., Zhang H., Guan C., Thulasidas M., Hoshi Y., Ishikawa A., Shimizu K., Birbaumer N. Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a brain-computer interface. Neuroimage 2007; 34(4): 1416–1427, https://doi.org/10.1016/j.neuroimage.2006.11.005.
- Coyle S.M., Ward T.E., Markham C.M. Brain-computer interface using a simplified functional near-infrared spectroscopy system. J Neural Eng 2007; 4(3): 219–226, https://doi.org/10.1088/1741-2560/4/3/007.
- Hramov A.E., Grubov V., Badarin A., Maksimenko V.A., Pisarchik A.N. Functional near-infrared spectroscopy for the classification of motor-related brain activity on the sensor-level. Sensors (Basel) 2020; 20(8): 2362, https://doi.org/10.3390/s20082362.
- Isaev M.R., Bobrov P.D. Effects of selection of the learning set formation strategy and filtration method on the effectiveness of a BCI based on near infrared spectrometry. Neurosci Behav Physi 2023; 53: 373–380, https://doi.org/10.1007/s11055-023-01436-2.
- Tanamachi K., Kuwahara W., Okawada M., Sasaki S., Kaneko F. Relationship between resting-state functional connectivity and change in motor function after motor imagery intervention in patients with stroke: a scoping review. J Neuroeng Rehabil 2023; 20(1): 159, https://doi.org/10.1186/s12984-023-01282-w.
- Yan T., Liang W., Chan C.W.H., Shen Y., Liu S., Li M. Effects of motor imagery training on gait performance in individuals after stroke: a systematic review and meta-analysis. Disabil Rehabil 2025; 47(1): 47–61, https://doi.org/10.1080/09638288.2024.2337091.
- Villa-Berges E., Laborda Soriano A.A., Lucha-López O., Tricas-Moreno J.M., Hernández-Secorún M., Gómez-Martínez M., Hidalgo-García C. Motor imagery and mental practice in the subacute and chronic phases in upper limb rehabilitation after stroke: a systematic review. Occup Ther Int 2023; 2023: 3752889, https://doi.org/10.1155/2023/3752889.
- Karikari E., Koshechkin K.A. Review on brain-computer interface technologies in healthcare. Biophys Rev 2023; 15(5): 1351–1358, https://doi.org/10.1007/s12551-023-01138-6.
- Valeriani D., Santoro F., Ienca M. The present and future of neural interfaces. Front Neurorobot 2022; 16: 953968, https://doi.org/10.3389/fnbot.2022.953968.
- Peksa J., Mamchur D. State-of-the-art on brain-computer interface technology. Sensors (Basel) 2023; 23(13): 6001, https://doi.org/10.3390/s23136001.
- The Brain-Computer Interface (BCI) Society website. BCI definition. URL: https://bcisociety.org/bci-definition/ (accessed 2024-05-30).
- Mokienko O.A., Lyukmanov R.K., Bobrov P.D., Suponeva N.A., Piradov M.A. Brain-computer interfaces for upper limb motor recovery after stroke: current status and development prospects (review). Sovremennye tehnologii v medicine 2023; 15(6): 63–73, https://doi.org/10.17691/stm2023.15.6.07.
- Mihara M., Miyai I., Hattori N., Hatakenaka M., Yagura H., Kawano T., Okibayashi M., Danjo N., Ishikawa A., Inoue Y., Kubota K. Neurofeedback using real-time near-infrared spectroscopy enhances motor imagery related cortical activation. PLoS One 2012; 7(3): e32234, https://doi.org/10.1371/journal.pone.0032234.
- Kober S.E., Wood G., Kurzmann J., Friedrich E.V., Stangl M., Wippel T., Väljamäe A., Neuper C. Near-infrared spectroscopy based neurofeedback training increases specific motor imagery related cortical activation compared to sham feedback. Biol Psychol 2014; 95: 21–30, https://doi.org/10.1016/j.biopsycho.2013.05.005.
- Ota Y., Takamoto K., Urakawa S., Nishimaru H., Matsumoto J., Takamura Y., Mihara M., Ono T., Nishijo H. Motor imagery training with neurofeedback from the frontal pole facilitated sensorimotor cortical activity and improved hand dexterity. Front Neurosci 2020; 14: 34, https://doi.org/10.3389/fnins.2020.00034.
- Fujimoto H., Mihara M., Hattori N., Hatakenaka M., Yagura H., Kawano T., Miyai I., Mochizuki H. Neurofeedback-induced facilitation of the supplementary motor area affects postural stability. Neurophotonics 2017; 4(4): 045003, https://doi.org/10.1117/1.NPh.4.4.045003.
- Wang Z., Zhou Y., Chen L., Gu B., Yi W., Liu S., Xu M., Qi H., He F., Ming D. BCI monitor enhances electroencephalographic and cerebral hemodynamic activations during motor training. IEEE Trans Neural Syst Rehabil Eng 2019; 27(4): 780–787, https://doi.org/10.1109/TNSRE.2019.2903685.
- Mihara M., Hattori N., Hatakenaka M., Yagura H., Kawano T., Hino T., Miyai I. Near-infrared spectroscopy-mediated neurofeedback enhances efficacy of motor imagery-based training in poststroke victims: a pilot study. Stroke 2013; 44(4): 1091–1098, https://doi.org/10.1161/STROKEAHA.111.674507.
- Rieke J.D., Matarasso A.K., Yusufali M.M., Ravindran A., Alcantara J., White K.D., Daly J.J. Development of a combined, sequential real-time fMRI and fNIRS neurofeedback system to enhance motor learning after stroke. J Neurosci Methods 2020; 341: 108719, https://doi.org/10.1016/j.jneumeth.2020.108719.
- Mihara M., Fujimoto H., Hattori N., Otomune H., Kajiyama Y., Konaka K., Watanabe Y., Hiramatsu Y., Sunada Y., Miyai I., Mochizuki H. Effect of neurofeedback facilitation on poststroke gait and balance recovery: a randomized controlled trial. Neurology 2021; 96(21): e2587–e2598, https://doi.org/10.1212/WNL.0000000000011989.
- Lyukmanov R.Kh., Isaev M.R., Mokienko O.A., Bobrov P.D., Ikonnikova E.S., Cherkasova A.N., Suponeva N.A. Brain-computer interface using functional near-infrared spectroscopy for post-stroke motor rehabilitation: case series. Annals of Clinical and Experimental Neurology 2023; 17(4): 82–88, https://doi.org/10.54101/ACEN.2023.4.10.
- Isaev M.R., Mokienko O.A., Lyukmanov R.K., Ikonnikova E.S., Cherkasova A.N., Suponeva N.A., Piradov M.A., Bobrov P.D. A multiple session dataset of NIRS recordings from stroke patients controlling brain-computer interface. Scientific Data 2024; 11: 1168, https://doi.org/10.1038/s41597-024-04012-6.
- Mokienko O.A., Lyukmanov R.K., Bobrov P.D., Isaev M.R., Ikonnikova E.S., Cherkasova А.N., Suponeva N.A., Piradov M.A. Brain-computer interfaces based on near-infrared spectroscopy and electroencephalography registration in post-stroke rehabilitation: a comparative study. Neurology, Neuropsychiatry, Psychosomatics 2024; 16(5): 17–23, https://doi.org/10.14412/2074-2711-2024-5-17-23.
- Poydasheva A.G., Bakulin I.S., Zabirova A.Kh., Kirichenko O.A., Suponeva N.A., Piradov M.A. Therapeutic transcranial magnetic stimulation for post-stroke paresis: the need for a differentiated approach. Nervnyye bolezni 2023; 4: 29–34, https://doi.org/10.24412/2226-0757-2023-13043.
- Zhong G., Yang Z., Jiang T. Precise modulation strategies for transcranial magnetic stimulation: advances and future directions. Neurosci Bull 2021; 37(12): 1718–1734, https://doi.org/10.1007/s12264-021-00781-x.
- Tang Z., Liu T., Han K., Liu Y., Su W., Wang R., Zhang H. The effects of rTMS on motor recovery after stroke: a systematic review of fMRI studies. Neurol Sci 2024; 45(3): 897–909, https://doi.org/10.1007/s10072-023-07123-x.
- Siebner H.R., Funke K., Aberra A.S., Antal A., Bestmann S., Chen R., Classen J., Davare M., Di Lazzaro V., Fox P.T., Hallett M., Karabanov A.N., Kesselheim J., Beck M.M., Koch G., Liebetanz D., Meunier S., Miniussi C., Paulus W., Peterchev A.V., Popa T., Ridding M.C., Thielscher A., Ziemann U., Rothwell J.C., Ugawa Y. Transcranial magnetic stimulation of the brain: what is stimulated? — A consensus and critical position paper. Clin Neurophysiol 2022; 140: 59–97, https://doi.org/10.1016/j.clinph.2022.04.022.
- Wang C., Zhang Q., Zhang L., Zhao D., Xu Y., Liu Z., Wu C., Wu S., Yong M., Wu L. Comparative efficacy of different repetitive transcranial magnetic stimulation protocols for lower extremity motor function in stroke patients: a network meta-analysis. Front Neurosci 2024; 18: 1352212, https://doi.org/10.3389/fnins.2024.1352212.
- Zhu M., Huang S., Chen W., Pan G., Zhou Y. The effect of transcranial magnetic stimulation on cognitive function in post-stroke patients: a systematic review and meta-analysis. BMC Neurol 2024; 24(1): 234, https://doi.org/10.1186/s12883-024-03726-9.
- Liu X., Li H., Yang S., Xiao Z., Li Q., Zhang F., Ma J. Efficacy of repetitive transcranial magnetic stimulation on post-stroke cognitive impairment: a systematic and a network meta-analysis. Int J Geriatr Psychiatry 2024; 39(7): e6117, https://doi.org/10.1002/gps.6117.
- Cheng J., Jiang Y., Rao T., Yang Y., Liu Y., Zhan Y., Yang S. Repetitive transcranial magnetic stimulation for post-stroke non-fluent aphasia: a systematic review and meta-analysis of randomized controlled trials. Front Neurol 2024; 15: 1348695, https://doi.org/10.3389/fneur.2024.1348695.
- Han D., Cheng J., Chen Y., Du H., Lin Z., Zhong R., Liu Z. Evidence for intermittent theta burst transcranial magnetic stimulation for dysphagia after stroke: a systematic review and meta-analysis. Dysphagia 2025; 40(1): 54–65, https://doi.org/10.1007/s00455-024-10729-8.
- Mayor R.S., Ferreira N.R., Lanzaro C., Castelo-Branco M., Valentim A., Donato H., Lapa T. Noninvasive transcranial brain stimulation in central post-stroke pain: a systematic review. Scand J Pain 2024; 24(1): 10.1515/sjpain-2023-0130, https://doi.org/10.1515/sjpain-2023-0130.
- Liu Y., Miao R., Zou H., Hu Q., Yin S., Zhu F. Repetitive transcranial magnetic stimulation in central post-stroke pain: a meta-analysis and systematic review of randomized controlled trials. Front Neurosci 2024; 18: 1367649, https://doi.org/10.3389/fnins.2024.1367649.
- Wang T., Liu X., Wu X., Fan Y., Lv Y., Chen B. High-frequency rTMS of the left dorsolateral prefrontal cortex for post-stroke depression: a systematic review and meta-analysis. Clin Neurophysiol 2024; 157: 130–141, https://doi.org/10.1016/j.clinph.2023.11.019.
- Nettekoven C., Volz L.J., Leimbach M., Pool E.M., Rehme A.K., Eickhoff S.B., Fink G.R., Grefkes C. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. Neuroimage 2015; 118: 209–218, https://doi.org/10.1016/j.neuroimage.2015.06.004.
- Curtin A., Tong S., Sun J., Wang J., Onaral B., Ayaz H. A systematic review of integrated functional near-infrared spectroscopy (fNIRS) and transcranial magnetic stimulation (TMS) studies. Front Neurosci 2019; 13: 84, https://doi.org/10.3389/fnins.2019.00084.
- Jiang S., Carpenter L.L., Jiang H. Optical neuroimaging: advancing transcranial magnetic stimulation treatments of psychiatric disorders. Vis Comput Ind Biomed Art 2022; 5(1): 22, https://doi.org/10.1186/s42492-022-00119-y.
- Hara T., Abo M., Kakita K., Mori Y., Yoshida M., Sasaki N. The effect of selective transcranial magnetic stimulation with functional near-infrared spectroscopy and intensive speech therapy on individuals with post-stroke aphasia. Eur Neurol 2017; 77(3–4): 186–194, https://doi.org/10.1159/000457901.
- Chang P.W., Lu C.F., Chang S.T., Tsai P.Y. functional near-infrared spectroscopy as a target navigator for rTMS modulation in patients with hemiplegia: a randomized control study. Neurol Ther 2022; 11(1): 103–121, https://doi.org/10.1007/s40120-021-00300-0.
- A study on the effectiveness of upper extremity rehabilitation training using brain-machine interface biofeedback in stroke patients with hemiplegia. 2020. URL: https://clinicaltrials.gov/study/NCT04290377 (study start 2020.02.25).
- Asgher U., Khan M.J., Asif Nizami M.H., Khalil K., Ahmad R., Ayaz Y., Naseer N. Motor training using mental workload (MWL) with an assistive soft exoskeleton system: a functional near-infrared spectroscopy (fNIRS) study for brain-machine interface (BMI). Front Neurorobot 2021; 15: 605751, https://doi.org/10.3389/fnbot.2021.605751.
- Khan R.A., Naseer N., Qureshi N.K., Noori F.M., Nazeer H., Khan M.U. fNIRS-based neurorobotic interface for gait rehabilitation. J Neuroeng Rehabil 2018; 15(1): 7, https://doi.org/10.1186/s12984-018-0346-2.
- Xie Y.L., Yang Y.X., Jiang H., Duan X.Y., Gu L.J., Qing W., Zhang B., Wang Y.X. Brain-machine interface-based training for improving upper extremity function after stroke: a meta-analysis of randomized controlled trials. Front Neurosci 2022; 16: 949575, https://doi.org/10.3389/fnins.2022.949575.
- Bai Z., Fong K.N.K., Zhang J.J., Chan J., Ting K.H. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: a systematic review and meta-analysis. J Neuroeng Rehabil 2020; 17(1): 57, https://doi.org/10.1186/s12984-020-00686-2.
- Mansour S., Ang K.K., Nair K.P.S., Phua K.S., Arvaneh M. Efficacy of brain-computer interface and the impact of its design characteristics on poststroke upper-limb rehabilitation: a systematic review and meta-analysis of randomized controlled trials. Clin EEG Neurosci 2022; 53(1): 79–90, https://doi.org/10.1177/15500594211009065.
- Nojima I., Sugata H., Takeuchi H., Mima T. Brain-computer interface training based on brain activity can induce motor recovery in patients with stroke: a meta-analysis. Neurorehabil Neural Repair 2022; 36(2): 83–96, https://doi.org/10.1177/15459683211062895.
- Kotov S.V., Slyunkova E.V., Borisova V.A., Isakova E.V. Effectiveness of brain-computer interfaces and cognitive training using computer technologies in restoring cognitive functions in patients after stroke. Zhurnal nevrologii i psikhiatrii im. S.S. Korsakova 2022; 122(12–2): 67–75, https://doi.org/10.17116/jnevro202212212267.
- Borisova V.A., Isakova E.V., Kotov S.V. Possibilities of the brain-computer interface in the correction of post-stroke cognitive impairments. Zhurnal nevrologii i psikhiatrii im. S.S. Korsakova 2022; 122(12): 60–66, https://doi.org/10.17116/jnevro202212212260.
- Kober S.E., Gressenberger B., Kurzmann J., Neuper C., Wood G. Voluntary modulation of hemodynamic responses in swallowing related motor areas: a near-infrared spectroscopy-based neurofeedback study. PLoS One 2015; 10(11): e0143314, https://doi.org/10.1371/journal.pone.0143314.
- Kober S.E., Hinterleitner V., Bauernfeind G., Neuper C., Wood G. Trainability of hemodynamic parameters: a near-infrared spectroscopy based neurofeedback study. Biol Psychol 2018; 136: 168–180, https://doi.org/10.1016/j.biopsycho.2018.05.009.
- Kober S.E., Spörk R., Bauernfeind G., Wood G. Age-related differences in the within-session trainability of hemodynamic parameters: a near-infrared spectroscopy-based neurofeedback study. Neurobiol Aging 2019; 81: 127–137, https://doi.org/10.1016/j.neurobiolaging.2019.05.022.
- Independent use of brain measurement-based rehabilitation system by stroke survivors: a randomized open label phase 1 clinical trial with blinded evaluation. 2021. URL: https://clinicaltrials.gov/study/NCT05016193 (study start 2021.08.01).
- Portable method of motor rehabilitation using functional near-infrared spectroscopy-based brain-computer-interface to augment post-stroke recovery (fNIRS-PROMOTE-recovery). 2022. URL: https://clinicaltrials.gov/study/NCT05258591 (study start 2022.02.23).
- Shin J., von Lühmann A., Kim D.W., Mehnert J., Hwang H.J., Müller K.R. Simultaneous acquisition of EEG and NIRS during cognitive tasks for an open access dataset. Sci Data 2018; 5: 180003, https://doi.org/10.1038/sdata.2018.3.
- Shin J., von Luhmann A., Blankertz B., Kim D.W., Jeong J., Hwang H.J., Muller K.R. Open access dataset for EEG+NIRS single-trial classification. IEEE Trans Neural Syst Rehabil Eng 2017; 25(10): 1735–1745, https://doi.org/10.1109/TNSRE.2016.2628057.
- von Lühmann A., Li X., Gilmore N., Boas D.A., Yücel M.A. Open access multimodal fNIRS resting state dataset with and without synthetic hemodynamic responses. Front Neurosci 2020; 14: 579353, https://doi.org/10.3389/fnins.2020.579353.
- Bak S., Park J., Shin J., Jeong J. Open-access fNIRS dataset for classification of unilateral finger- and foot-tapping. Electronics 2019; 8(12), https://doi.org/10.3390/electronics8121486.
- Khan H., Naseer N., Yazidi A., Eide P.K., Hassan H.W., Mirtaheri P. Analysis of human gait using hybrid EEG-fNIRS-based BCI system: a review. Front Hum Neurosci 2021; 14: 613254, https://doi.org/10.3389/fnhum.2020.613254.
- Yin X., Xu B., Jiang C., Fu Y., Wang Z., Li H., Shi G. A hybrid BCI based on EEG and fNIRS signals improves the performance of decoding motor imagery of both force and speed of hand clenching. J Neural Eng 2015; 12(3): 036004, https://doi.org/10.1088/1741-2560/12/3/036004.
- Ahn S., Jun S.C. Multi-modal integration of EEG-fNIRS for brain-computer interfaces — current limitations and future directions. Front Hum Neurosci 2017; 11: 503, https://doi.org/10.3389/fnhum.2017.00503.
- Bobrov P.D., Isaev M.R., Korshakov A.V., Oganesyan V.V., Kerechanin J.V., Popodko A.I., Frolov A.A. Sources of electrophysiological and foci of hemodynamic brain activity most relevant for controlling a hybrid brain-computer interface based on classification of EEG patterns and near-infrared spectrography signals during motor imagery. Hum Physiol 2016; 42(3): 241–251, https://doi.org/10.1134/S036211971603004X.
- Favetta M., Romano A., Valè N., Cieslik B., Federico S., Girolami A., Mazzarotto D., Pregnolato G., Righetti A., Salvalaggio S., Castelli E., Smania N., Bargellesi S., Kiper P., Petrarca M. A scoping review of scientific concepts concerning motor recovery after stroke as employed in clinical trials. Front Neurol 2023; 14: 1221656, https://doi.org/10.3389/fneur.2023.1221656.
- Effects of repetitive transcranial magnetic stimulation based on hemodynamic activity for language recovery in early poststroke aphasia: randomized controlled trial. 2015. URL: https://clinicaltrials.gov/study/NCT02591719 (study start 2015.11).
- Efficacy of individualized repetitive transcranial magnetic stimulation based on cortical laterality for motor recovery in stroke patients. 2023. URL: https://clinicaltrials.gov/study/NCT05914038 (study start 2023.10.13).
- Sharma G., Roy Chowdhury S. Design of NIRS probe based on computational model to find out the optimal location for non-invasive brain stimulation. J Med Syst 2018; 42(12): 244, https://doi.org/10.1007/s10916-018-1039-x.
- Yang D., Shin Y.I., Hong K.S. Systemic review on transcranial electrical stimulation parameters and EEG/fNIRS Features for brain diseases. Front Neurosci 2021; 15: 629323, https://doi.org/10.3389/fnins.2021.629323.