Three-Dimensional Imaging of Human Myocardial Collagen Fibers Using Trypan Blue Staining
The aim of the study was to estimate the availability of trypan blue staining of human myocardium sections to investigate cardiac collagen fibers, in particular, their three-dimensional reconstructions.
Materials and Methods. Pretreated with phosphomolybdic acid sections of human myocardium (n=9) were stained with 1% aqueous trypan blue solution (Panreac, Spain) (patent application RU 2024/22564). Parallel sections were stained using the similar technique with 2% aqueous solution of aniline blue (Unisource Chemicals Pvt. Ltd., India). The obtained preparations were analyzed using a light microscope Leica DM750 (Leica, Germany) and a confocal laser microscope LSM800 (Carl Zeiss AG, Germany). Three-dimensional reconstruction was performed using software ZEN-2012 (Carl Zeiss AG, Germany).
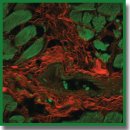
Results. Trypan blue appeared to stain collagen fibers selectively. Loose thick fibers were stained more intensively than thin fibers, which were not available for detailed examination with bright-field microscopy. The cytoplasm of cardiomyocytes remained unstained. Aniline blue appeared to stain thin fibers relatively more intensively, and its non-specifically stained the cytoplasm of cardiomyocytes.
Fluorescence of collagen fibers stained with trypan blue was excited with a 640 nm laser. Autofluorescence of the cytoplasm of cardiomyocytes was excited with a 488 nm laser. Three-dimensional reconstruction of endomysium showed that it was a net. Therefore, it became possible to describe the lower-order structures being the part of thick fibers. Three-dimensional reconstruction enabled to distinguish the orientation of the fibers and their cross section differentiating it from tangential one.
Conclusion. The study findings showed trypan blue staining of human cardiac section to be an effective method to reveal collagen fibers, study their structure with bright-field microscopy using confocal laser microscopy and perform detailed three-dimensional reconstructions. The advantages of the method compared with other techniques of three-dimensional imaging of collagen fibers consist in the accessibility of reagents, high staining selectivity of the connective tissue matrix, the ability to obtain contrast and detailed images of the studied objects with high resolution.
- Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 2014; 15(12): 802–812, https://doi.org/10.1038/nrm3896.
- Smoluk L., Protsenko Y. Viscoelastic properties of the papillary muscle: experimental and theoretical study. Acta Bioeng Biomech 2012; 14(4): 37–44.
- Orgel J.P., San Antonio J.D., Antipova O. Molecular and structural mapping of collagen fibril interactions. Connect Tissue Res 2011; 52(1): 2–17, https://doi.org/10.3109/03008207.2010.511353.
- Potekhina Y.P., Filatova A.I., Tregubova E.S., Mokhov D.E. Mechanosensitivity of cells and its role in the regulation of physiological functions and the implementation of physiotherapeutic effects (review). Sovremennye tehnologii v medicine 2021; 12(4): 77–89, https://doi.org/10.17691/stm2020.12.4.10.
- Meyers V.E., Zayzafoon M., Douglas J.T., McDonald J.M. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res 2005; 20(10): 1858–1866, https://doi.org/10.1359/JBMR.050611.
- Gilbert P.M., Havenstrite K.L., Magnusson K.E., Sacco A., Leonardi N.A., Kraft P., Nguyen N.K., Thrun S., Lutolf M.P., Blau H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010; 329(5995): 1078–1081, https://doi.org/10.1126/science.1191035.
- Tondon A., Kaunas R. The direction of stretch-induced cell and stress fiber orientation depends on collagen matrix stress. PLoS One 2014; 9(2): e89592, https://doi.org/10.1371/journal.pone.0089592.
- Bishop J.E., Rhodes S., Laurent G.J., Low R.B., Stirewalt W.S. Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res 1994; 28(10): 1581–1585, https://doi.org/10.1093/cvr/28.10.1581.
- Sanchez-Quintana D., Climent V., Ho S.Y., Anderson R.H. Myoarchitecture and connective tissue in hearts with tricuspid atresia. Heart 1999; 81(2): 182–191, https://doi.org/10.1136/hrt.81.2.182.
- Mishra P.K., Givvimani S., Chavali V., Tyagi S.C. Cardiac matrix: a clue for future therapy. Biochim Biophys Acta 2013; 1832(12): 2271–2276, https://doi.org/10.1016/j.bbadis.2013.09.004.
- González A., López B., Ravassa S., San José G., Díez J. The complex dynamics of myocardial interstitial fibrosis in heart failure. Focus on collagen cross-linking. Biochim Biophys Acta Mol Cell Res 2019; 1866(9): 1421–1432, https://doi.org/10.1016/j.bbamcr.2019.06.001.
- Perestrelo A.R., Silva A.C., Oliver-De La Cruz J., Martino F., Horváth V., Caluori G., Polanský O., Vinarský V., Azzato G., de Marco G., Žampachová V., Skládal P., Pagliari S., Rainer A., Pinto-do-Ó P., Caravella A., Koci K., Nascimento D.S., Forte G. Multiscale analysis of extracellular matrix remodeling in the failing heart. Circ Res 2021; 128(1): 24–38, https://doi.org/10.1161/CIRCRESAHA.120.317685.
- Nikitina I.A., Razenkova V.A., Fedorova E.A., Kirik O.V., Korzhevskii D.E. Technology of combined identification of macrophages and collagen fibers in liver samples. Sovremennye tehnologii v medicine 2024; 16(3): 24–29, https://doi.org/10.17691/stm2024.16.3.03.
- Junqueira L.C., Bignolas G., Brentani R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 1979; 11(4): 447–455, https://doi.org/10.1007/BF01002772.
- Dolber P.C., Spach M.S. Conventional and confocal fluorescence microscopy of collagen fibers in the heart. J Histochem Cytochem 1993; 41(3): 465–469, https://doi.org/10.1177/41.3.7679127.
- Kim H.J., Kim J., Choi J., Sun W. Chemical fluorescence-based dye staining for 3-dimensional histopathology analysis. Anim Cells Syst (Seoul) 2022; 26(2): 45–51, https://doi.org/10.1080/19768354.2022.2049641.
- Cox G., Kable E., Jones A., Fraser I., Manconi F., Gorrell M.D. 3-dimensional imaging of collagen using second harmonic generation. J Struct Biol 2003; 141(1): 53–62, https://doi.org/10.1016/s1047-8477(02)00576-2.
- Wülfers E.M., Greiner J., Giese M., Madl J., Kroll J., Stiller B., Kohl P., Rog-Zielinska E.A., Fürniss H.E. Quantitative collagen assessment in right ventricular myectomies from patients with tetralogy of Fallot. Europace 2021; 23(23 Suppl 1): i38–i47, https://doi.org/10.1093/europace/euaa389.
- Zanatta G., Steffens D., Braghirolli D.I., Fernandes R.A., Netto C.A., Pranke P. Viability of mesenchymal stem cells during electrospinning. Braz J Med Biol Res 2012; 45(2): 125–130, https://doi.org/10.1590/s0100-879x2011007500163.
- Dada V.K., Sharma N., Sudan R., Sethi H., Dada T., Pangtey M.S. Anterior capsule staining for capsulorhexis in cases of white cataract: comparative clinical study. J Cataract Refract Surg 2004; 30(2): 326–333, https://doi.org/10.1016/S0886-3350(03)00573-X.
- King L.S. Vital staining of the connective tissues. J Exp Med 1938; 68(1): 63–72, https://doi.org/10.1084/jem.68.1.63.
- Srivastava V.K., Singh R.K., Malhotra S.N., Singh A. To evaluate cytotoxicity of resin-based restorative materials on human lymphocytes by trypan blue exclusion test: an in vitro study. Int J Clin Pediatr Dent 2010; 3(3): 147–152, https://doi.org/10.5005/jp-journals-10005-1070.
- Hammoudeh S.M., Hammoudeh A.M., Hamoudi R. High-throughput quantification of the effect of DMSO on the viability of lung and breast cancer cells using an easy-to-use spectrophotometric trypan blue-based assay. Histochem Cell Biol 2019; 152(1): 75–84, https://doi.org/10.1007/s00418-019-01775-7.
- Procel N., Camacho K., Verboven E., Baroja I., Guerrero P.A., Hillen H., Estrella-García C., Vizcaíno-Rodríguez N., Sansores-Garcia L., Santamaría-Naranjo A., Romero-Carvajal A., Caicedo A., Halder G., Moya I.M. In vivo tracking and 3D mapping of cell death in regeneration and cancer using trypan blue. Cells 2024; 13(16): 1379, https://doi.org/10.3390/cells13161379.
- Teba F.A., Mohr A., Eckardt C., Wong D., Kusaka S., Joondeph B.C., Feron E.J., Stalmans P., Van Overdam K., Melles G.R. Trypan blue staining in vitreoretinal surgery. Ophthalmology 2003; 110(12): 2409–2412, https://doi.org/10.1016/s0161-6420(03)00716-4.
- Fridman G., Rizzuti A.E., Liao J., Rolain M., Deutsch J.A., Kaufman S.C. Trypan blue as a surgical adjunct in pediatric cataract surgery. J Cataract Refract Surg 2016; 42(12): 1774–1778, https://doi.org/10.1016/j.jcrs.2016.10.012.
- Jackson T.L., Kwan A.S., Laidlaw A.H., Aylward W. Identification of retinal breaks using subretinal trypan blue injection. Ophthalmology 2007; 114(3): 587–590, https://doi.org/10.1016/j.ophtha.2006.05.079.
- Parker J.S., Parker A., Parker J.S. Trypan blue-assisted microinvasive glaucoma surgery. J Cataract Refract Surg 2017; 43(12): 1613, https://doi.org/10.1016/j.jcrs.2017.10.031.
- Adams C.W., Bayliss O.B. Permeability of inner and outer layers of rat and rabbit aortic wall. Two new microscopic test with trypan blue. Atherosclerosis 1977; 26(4): 419–426, https://doi.org/10.1016/0021-9150(77)90112-5.
- Masson P. Some histological methods. Trichrome stainings and their preliminary technique. J Tech Methods 1929; 12: 75–90.
- Mallory F.B. A contribution to staining methods: I. A differential stain for connective-tissue fibrillae and reticulum. II. Chloride of iron haematoxylin for nuclei and fibrin. III. Phosphotungstic acid haematoxylin for neuroglia fibres. J Exp Med 1900; 5(1): 15–20, https://doi.org/10.1084/jem.5.1.15.
- Heidenhain M. Über die Mallorysche Bindegewebsfärbung mit Karmin und Azokarmin als Vorfarben. Z Wiss Mikrosk 1915; 32: 361–372.
- Slinchenko N.Z. Rapid and stable staining of connective tissue, hyalin, fibrin and fibrinoids. Arkhiv patologii 1964; 26: 84.
- Puchtler H., Isler H. The effect of phosphomolybdic acid on the stainability of connective tissues by various dyes. J Histochem Cytochem 1958; 6(4): 265–270, https://doi.org/10.1177/6.4.265.
- Everett M.M., Miller W.A. The role of phosphotungstic and phosphomolybdic acids in connective tissue staining. I. Histochemical studies. Histochem J 1974; 6(1): 25–34, https://doi.org/10.1007/BF01011535.
- Weis S.M., Emery J.L., Becker K.D., McBride D.J. Jr, Omens J.H., McCulloch A.D. Myocardial mechanics and collagen structure in the osteogenesis imperfecta murine (oim). Circ Res 2000; 87(8): 663–669, https://doi.org/10.1161/01.res.87.8.663.
- Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol 2002; 65(2): 109–126, https://doi.org/10.1679/aohc.65.109.
- Bancelin S., Aimé C., Gusachenko I., Kowalczuk L., Latour G., Coradin T., Schanne-Klein M.C. Determination of collagen fibril size via absolute measurements of second-harmonic generation signals. Nat Commun 2014; 5: 4920, https://doi.org/10.1038/ncomms5920.
- Ajeti V., Nadiarnykh O., Ponik S.M., Keely P.J., Eliceiri K.W., Campagnola P.J. Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: implications for probing stromal alterations in human breast cancer. Biomed Opt Express 2011; 2(8): 2307–2316, https://doi.org/10.1364/BOE.2.002307.
- Cicchi R., Pavone F.S. Probing collagen organization: practical guide for second-harmonic generation (SHG) imaging. Methods Mol Biol 2017; 1627: 409–425, https://doi.org/10.1007/978-1-4939-7113-8_27.










