The Original Mouse Models of Glioblastoma: Analysis of Pathophysiological Characteristics of Transplanted Tumor Tissue
The aim of this study was to morphologically, molecularly, and immunologically characterize two new transplantable glioblastoma (GB) tissue models, designated M2 GB and M6 GB.
Materials and Methods. Two new chemically induced, easily transplantable tissue mouse models of high-grade glioma have been created and characterized. M2 GB and M6 GB tissues were orthotopically transplanted to immunocompetent C57BL/6 mice. The clinical and morphological characteristics of tumor growth, as well as the intratumoral immune response and target gene expression were assessed.
Results. Clinical manifestations of M2 GB and M6 GB growth in mice include motility disorders, cachexia, and priapism. Morphologically, M2 GB and M6 GB are characterized by diffuse proliferation, cellular and nuclear polymorphism, and high mitotic activity with pathological mitotic patterns corresponding to the aggressive nature of the mentioned tumors. Both tumors were significantly infiltrated with CD3+ T lymphocytes (~32%) and F4/80+ macrophages (~28–50%). M2 GB showed a higher content of F4/80+ macrophages compared to M6 GB. The Cdkn2a, S100b, Mki67, Pten, Vegfa, Hif1a, Sox2, Abcb1, and Gfap genes were overexpressed in both tumors. Expression of the Cd133, Tp53, and Pdgfra genes was increased in M2 GB. High expression of Pi3k and Gdnf was seen in M6 GB. Expression of Cd44, Pi3k, Hif1a, Gdnf, and Egfr was higher in M6 GB tissues compared to M2 GB, whereas expression of Cdkn2a, Tp53, Cd133,and Pdgfra was higher in M2 GB tissues compared to M6 GB.
Conclusion. The M2 GB and M6 GB models of transplanted tissues reproduce key characteristics of human GB, including similar intracellular immune profiles, clinical and morphological features, and gene expression patterns, which are important for further research in neurological oncology. These models can be used to develop diagnostic and treatment methods and to study tumor genesis.
Introduction
Glioblastoma (GB) is the most aggressive tumor in the central nervous system in adults. Despite improved short-term survival, long-term treatment effects are still poor, and tumor recurrence rate is almost 100% [1]. Understanding the mechanisms of tumor initiation, progression, and evolution is critical for therapeutic approaches development. For this purpose, relevant preclinical models of GB are required. Mouse model is the most commonly used model for preclinical studies [2]. Allogeneic, xenograft, and genetically modified mouse tumor models are known, each having its advantages and limitations. Genetically modified models are expensive and time-consuming to create, and gene expression may be suppressed in later generations of animals [3]. Xenografts do not reflect early stochastic carcinogenesis, and the molecular biological characteristics of the host organism and tumor differ. In this model, it is not possible to assess the impact of the immune system on carcinogenesis, therapy, and immunotherapy [4]. Allogeneic models do not represent the many human tumor types with characteristic driver molecular abnormalities [5], but they are easy to model and use in large series of experiments. In translational studies of human brain glioma, a number of key factors should be considered: the molecular, biological, and pathophysiological characteristics of the glioma model and the model organism, as well as the local and systemic immune response and the clinical pattern of tumor growth. The response of the GB model to treatment should be similar to the human tumor response, and the tumor should be chemically and radiologically resistant [6]. Available GB models have unique characteristics that should be taken into account when planning experiments, though currently there is no model that accurately reproduces human GB [2, 7–9]. Low reproducibility of successful results in preclinical studies, the difficulty of their correct interpretation and translation into clinical practice emphasize the importance of the correct selection of experimental models.
The aim of this study was to morphologically, molecularly, and immunologically characterize two new transplantable glioblastoma tissue models, designated M2 GB and M6 GB.
The study demonstrated that transplantable tumor tissue models have advantages compared to commonly used cell models: a heterogeneous cellular composition and gene expression more similar to human tumors [10, 11].
Materials and Methods
The study was conducted on 54 mature male house mice (Mus musculus) of the C57BL/6 line (20–22 g). The animals were allocated as follows: 22 mice for primary M2 GB and M6 GB tumor tissue collection; 14 for tumor tissue revitalization; 12 for the core experiment, including 6 for M2 GB and 6 for M6 GB; and 6 intact animals. The animals were kept in cages with a 12-hour light/dark cycle with unlimited access to food and water. Surgical and culture procedures were performed under aseptic conditions. Animal experiments were carried out in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 1986). The study was approved by the Bioethics Commission (Protocol No. 29(5) dated November 8, 2021) of the Avtsyn Research Institute of Human Morphology of Petrovsky National Research Centre of Surgery (Russia). Animals with the first clinical signs of tumor growth (decreased activity, paralysis, weight loss, and weakness) were euthanized [12].
Induction of primary tumors with a carcinogen. Primary tumor tissues were obtained after trocar-assisted implantation of 1 mg of the solid carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) (Thermo Fisher Scientific, USA) into the right cerebral hemisphere of house mice (Mus musculus; n=22) under intraperitoneal anesthesia using Zoletil 100 of 0.25 mg/animal (Virbac, France) with Xylanite of 0.5 mg/animal (Interchemie Werken, Netherlands). 60–90 days after DMBA implantation, a range of central nervous system (CNS) tumors developed in mice. Tumor tissues with morphological features of GB (M2 GB and M6 GB) were selected for primary sequential transplantations (8– 10 transplantations).
After 8–10 consecutive transplantations of primary tumor tissue into mice, stable M2 GB and M6 GB tissue models with similar morphology and growth latency periods were obtained. The transplanted M2 GB and M6 GB tumor tissues strains were registered and are stored at –196°C in the cryostorage facility of the Avtsyn Research Institute of Human Morphology of Petrovsky National Research Centre of Surgery FSBSI. They are cataloged in the Registry of experimental tumors of the nervous system (http://ckp-rf.ru/usu/498710/). They are used in experimental neurological oncology.
Revitalization and transplantation of M2 GB and M6 GB tissue samples after cryopreservation. Before the core study, the tumor tissues were revitalized. For this purpose, an ampoule containing tumor tissue was thawed in warm water (39°C) and centrifuged for 7 min at 250 g. Then, the supernatant was removed, and the pellet was resuspended in glutamine-free culture medium (PanEco, Russia). ~10 μl of GB tissue (~4·105 cells) was injected intracerebrally using a syringe and needle (20G) with a stopper. The cells were transplanted into animals (n=14) under intraperitoneal anesthesia (Zoletil 100 of 0.25 mg/animal and Xylanit of 5 mg/animal).
Transplantation of M2 GB and M6 GB tissue samples after revitalization to study the survival and progressing development of M2 GB and M6 GB tissue samples. Grown GB tissues at the final stages of growth were transplanted within an hour into animals of the main experimental group, as described below.
After mechanical dissociation (pipetting) of the tumor tissue, cell viability was quantified in ~10 μl tissue samples (n=12). Cell viability was assessed using a 0.4% aqueous trypan blue solution (Servicebio, China). Cells were counted immediately after staining using a Goryaev chamber (MiniMed, Russia) under a light microscope (Carl Zeiss, Germany). Cell viability was at least 98%.
Mechanically crushed tissues of M2 GB (n=6) and M6 GB (n=6) with a volume of ~10 μl (~4·105 cells) were implanted into the brain of mice under intraperitoneal anesthesia with Zoletil 100 of 0.25 mg/animal and Xylanite of 0.5 mg/animal. The detailed procedure of tumor transplantation is described in the earlier publication [13]. However, in this case, the tumor was transplanted into the brain 2 mm to the right of the sagittal suture (sutura sagittalis) and 2 mm caudal to the coronal suture (sutura coronalis) to a depth of 2 mm in the corpus striatum region using a stereotaxic device (RWD, China); AP: +1 mm; ML: +2.0 mm lateral to bregma; DV: –2.0 mm relative to the skull surface. The injection rate was ~10 µl/10 s for both models.
MR images of the mouse brain were obtained intravitally on day 16 for M2 GB and on day 23 for M6 GB using a 7T tomographic scanner (BioSpec 70/30 USR; Bruker BioSpin, Germany) with a gradient amplitude of 105 mT/m. Isoflurane (Laboratorios Karizoo, Spain) was used for anesthesia. Axial T1-weighted images were acquired 15 min after intraperitoneal injection of gadobutol contrast agent (Schering, Germany) of 15 mg gadolinium per animal. Slice thickness was 0.8 mm, and the number of slices was up to 32.
Morphological examination of the brain of animals with GB was conducted at the terminal stages of tumor growth, specifically on days 17–19 for M2 GB and on days 24–26 for M6 GB after transplantation. This time frame corresponds to the onset of the first clinical symptoms in mice; death occurs within 1–2 days from the onset of the first clinical signs (decreased activity, paralysis, weight loss, and priapism). Animals were euthanized with Zoletil 100 of 10 mg/kg. Brains with GB (n=12) were fixed in 10% buffered formalin (BioVitrum, Russia). Tissue sections (7 μm thick) were prepared on a Microm HM 340 microtome (Thermo Fisher Scientific, Germany) and stained with hematoxylin and eosin (BioVitrum, Russia). Tissue samples for each GB strain were taken from at least 6 mice. Morphological changes were assessed using a light microscope (Carl Zeiss, Germany).
Gene expression was analyzed by real-time PCR. Total RNA was isolated using the RNeasy Plus Mini kit (Qiagen, USA) from 6 M2 GB tissue samples, 6 M6 GB tissue samples, and 6 intact brain samples (30 mg each). The samples were stored in RNA Later solution (Invitrogen, USA). The mRNA content was greater than 300 ng/μl (NanoPhotometer N50; Implen, Germany). cDNA was synthesized from the total RNA using the MMLV RT kit (Eurogen, Russia). PCR assay was performed using qPCRmix-HS SYBR reagents and the fluorescent intercalating stain SYBR Green I (Eurogen, Russia). Primers were selected using the Primer-BLAST online resource (USA) according to the generally accepted requirements. The selected primers (Table 1) were synthesized by Evrogen (Russia). The threshold cycle (Ct) method was used and relative gene expression was calculated according to the method [14] taking into account the recommendations [15]. The Gapdh gene was used as a control. mRNA expression was compared between M2 GB and M6 GB tumor samples and intact mouse brain tissue.
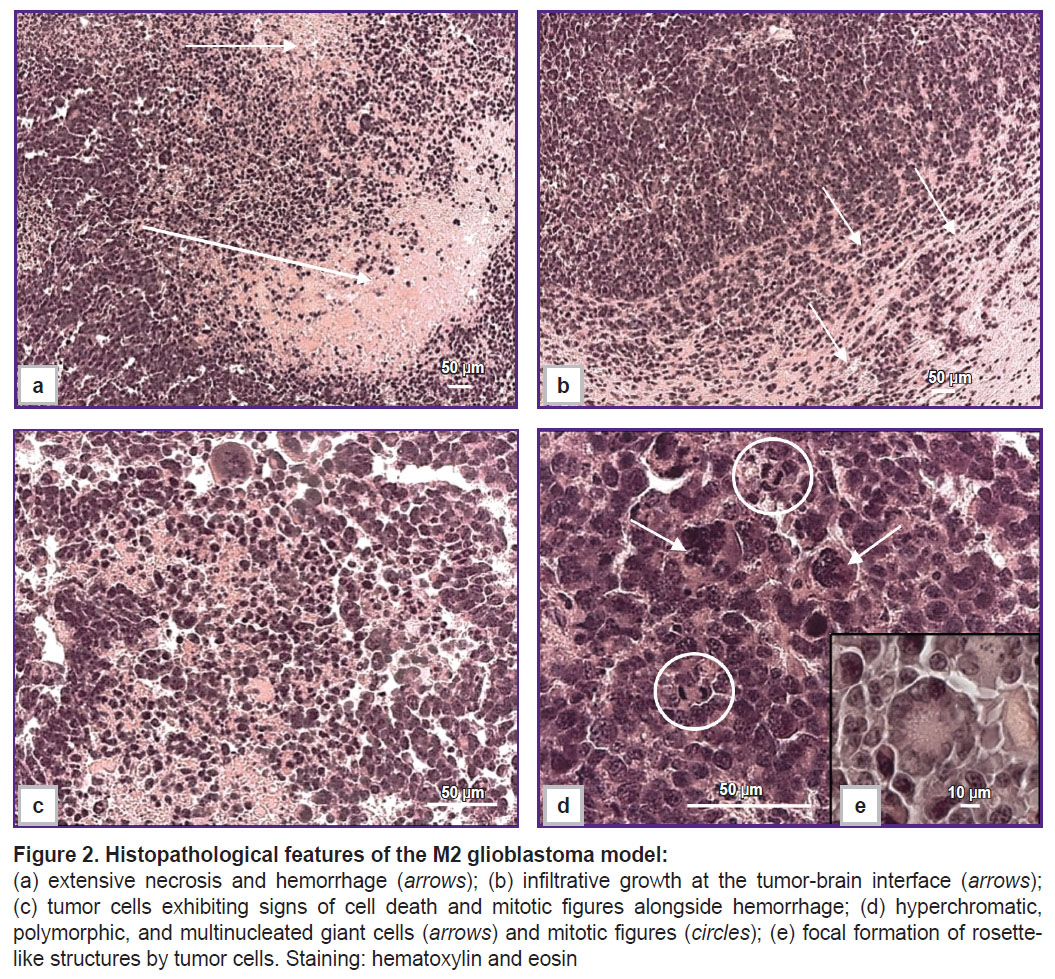
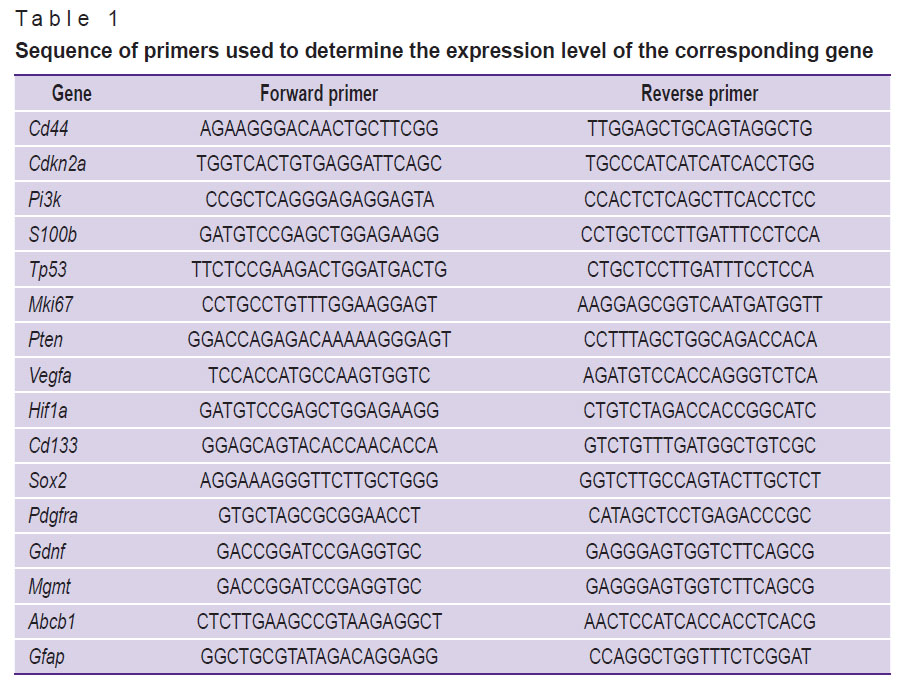 |
Table 1. Sequence of primers used to determine the expression level of the corresponding gene |
The relative number of lymphocytes and macrophages in the tumor tissue (106 cells; at least 5 samples for each GB strain) was assessed using a Cytomics FC 500 flow cytometer (Beckman Coulter, USA). Erythrocytes were lysed using OptiLyse C solution (eBioscience, USA). GB samples were incubated with antibodies to CD3-FITC (eBioscience, USA) and F4/80-PE (Miltenyi Biotec, Germany) for at least 30 min at room temperature. Samples without antibodies were used as controls for autofluorescence.
Statistical data analysis was conducted using the Statistica 10.0 package (StatSoft Inc, USA). Experimental data were characterized using the median (Me) and interquartile range [Q1; Q3]. Statistical differences were determined using the Kruskall–Wallis multiple comparison test. Dunn’s test was used for pairwise comparisons. Values were considered statistically significant at p≤0.05.
Results
The incidence of M2 GB and M6 GB formation was 95–100%. The average latent period of tumor growth in mice was 17–35 days for M2 GB and 23–34 days for M6 GB.
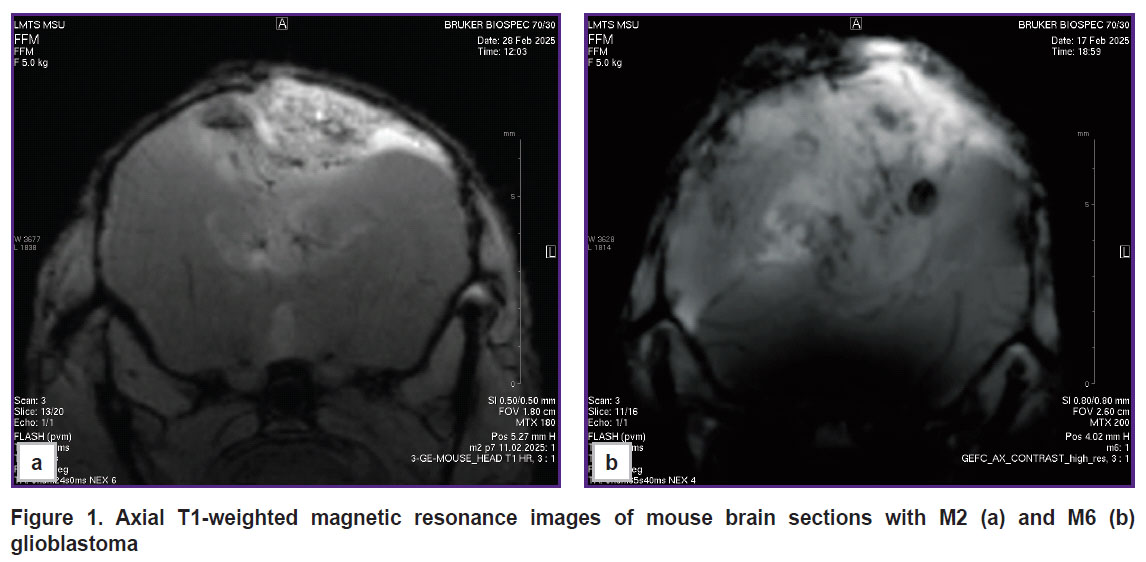
According to MRI of the brains of mice with M2 GB and M6 GB, the tumors were located in the right hemisphere; in the late stages, growth into the left hemisphere and a mass effect were observed: hemispheric asymmetry, compression with tissue deformation, and lateral displacement of midline brain structures (Figure 1).
|
Figure 1. Axial T1-weighted magnetic resonance images of mouse brain sections with M2 (a) and M6 (b) glioblastoma |
Morphologically, M2 GB cells (n=6) are large, exhibit high mitotic activity (Figure 2 (d)), and spread into the cortex and subcortical structures of the brain. The cells are polymorphic and atypical, with large nuclei, high cellularity, and a significant number of multinucleated giant cells (Figure 2 (c), (d)). Approximately 2% of mitoses were detected in the tumor, including pathological ones, which corresponded to two or more mitoses per 100 tumor cells. Approximately 2% of dying cells contained fragmented nuclei in the form of apoptotic bodies. Infiltrative growth (Figure 2 (b)), astroglial accumulation, and peritumoral edema were observed at the tumor–brain interface. Necrosis and hemorrhage were detected within the tumor parenchyma (Figure 2 (a)). Rosette-like tumor cell clusters (Figure 2 (e)) are a diagnostic feature of certain CNS tumors, such as medulloblastoma and neuroblastoma.
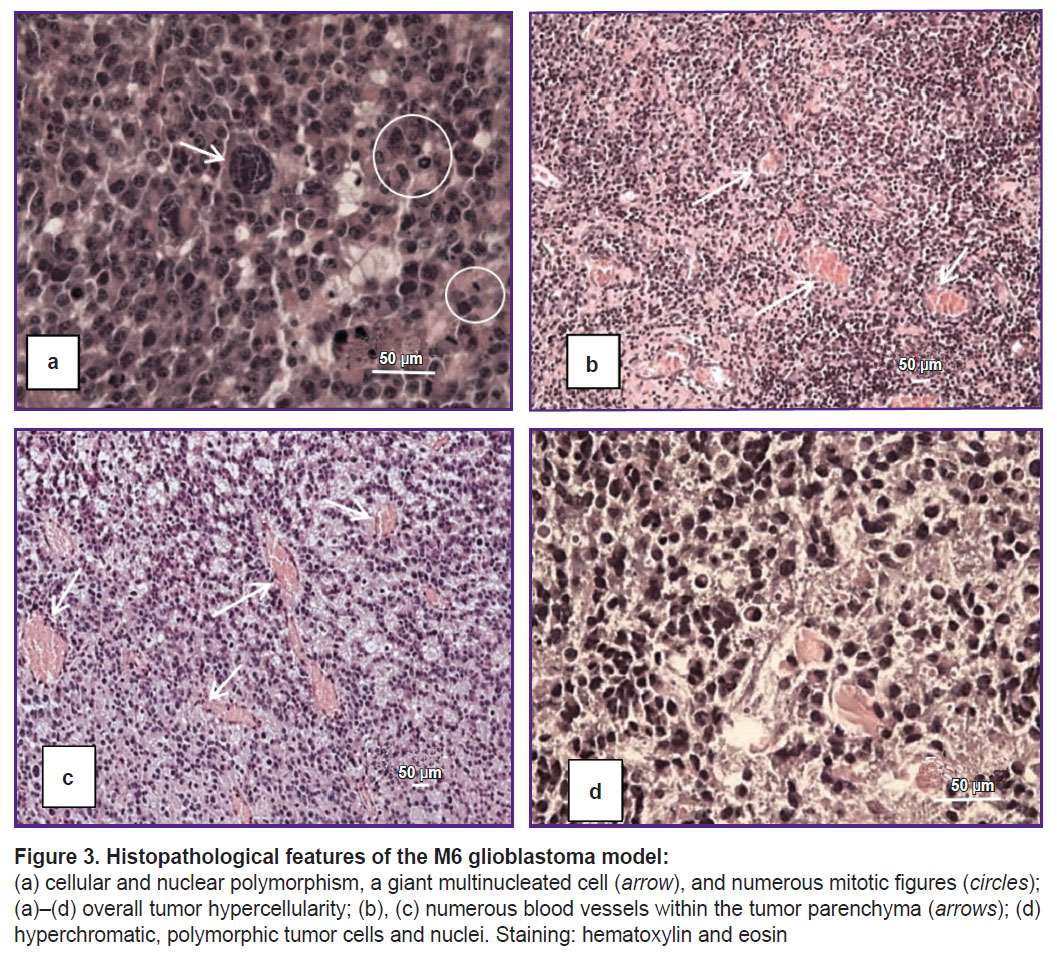
The M6 GB tumor cells (n=6) are polymorphic, with narrow cytoplasmic margins, predominantly giant morphology, and multiple nuclei (Figure 3 (a), (d)). Mitotic figures, including pathological forms, accounted for approximately 3% (see Figure 3 (a)), equivalent to three or more mitoses per 100 tumor cells. Approximately 3% of cells were identified as apoptotic bodies. The tumor parenchyma contained numerous vessels with distorted lumens, microvascular proliferation, and hemorrhages (Figure 3 (b)–(d)), along with several necrotic foci. Tumor growth was characterized by infiltrative and perivascular patterns.
Both M2 GB and M6 GB tissues were infiltrated by CD3+ T lymphocytes and F4/80+ macrophages. The relative numbers of T lymphocytes and macrophages in the M6 GB group (n=5) were 32.01 [8.90; 33.60]% and 28.4 [14.8; 28.4]%, respectively. In the M2 GB samples (n=5), the macrophage count was statistically significantly higher (p=0.04) at 50.3 [49.4; 51.2]% compared to 28.4 [14.8; 28.4]% in the M6 GB group.
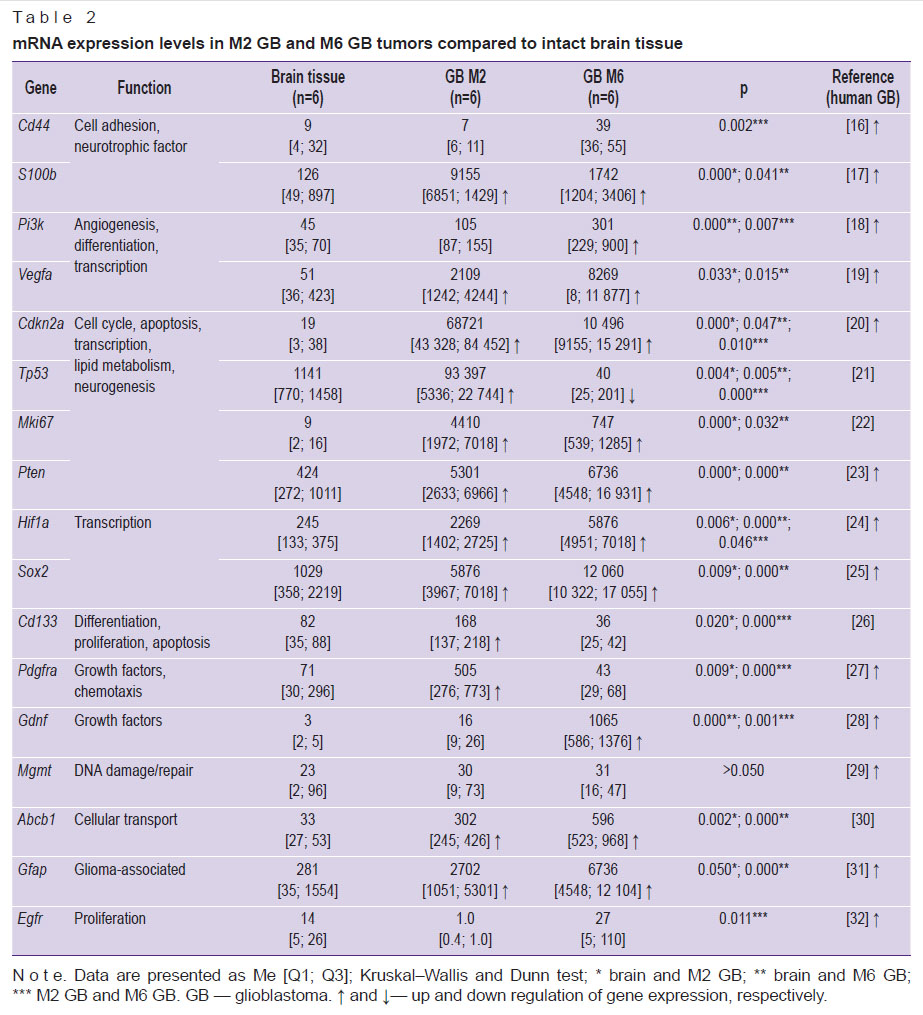
A comparative assessment of gene expression in the M2 GB and M6 GB models and intact brain tissue, alongside data from human GB [16–32], is presented in Table 2.
|
Table 2. mRNA expression levels in M2 GB and M6 GB tumors compared to intact brain tissue |
Discussion
Some studies noted that patients [33–36] and experimental animals [37–39] with brain tumors had a clinical pattern associated with decreased activity, paralysis, weight loss, and priapism. Similar symptoms in mice with M2 GB and M6 GB were observed in our study, indicating a terminal state in animals. The clinical manifestations of GB progression were associated with systemic metabolic alterations, including cachexia, coagulopathy, and signs of ischemic brain injury. Associated neurological symptoms included priapism and motor deficits.
Neuropathologists assign a tumor a high-grade malignancy based on 2 or more mitoses in the whole sample or 1 mitosis in a small biopsy [40]. This is consistent with the data on the high-grade malignancy of M2 GB and M6 GB received in this study.
Glioblastoma is an immunosuppressive tumor. Local and systemic immune dysfunction limit the efficacy of immunotherapy. Myeloid-derived suppressor cells and tumor-associated macrophages can inhibit T-cell infiltration and activation in gliomas [41]. Glioma cells and infiltrating immune cells evade immune surveillance by secreting immunosuppressive factors, such as IL-6, IL-10, TGF-β, and prostaglandin E2, whose expression is regulated by tumor-derived growth factors [42]. The difference in the abundance of T lymphocytes and macrophages between the M2 GB and M6 GB models is likely determined by the higher secretion of anti-inflammatory cytokines by M2 GB cells.
Tumor-infiltrating stromal cells, including macrophages/microglia, often exert a more potent immunosuppressive effect than the tumor cells themselves. Immunosuppression impacts the clonal composition of tumor cells and modulates gene expression [43]. An increased content of T lymphocytes in a tumor indicates an inflammatory tumor status, and a high number of F4/80+ indicates an immunosuppressive background in the tumor [44]. HIV-mediated immunosuppression is associated with an increased incidence of gliomas and the progression of low-grade glioma to GBs, which evidences a significant contribution of the immunosuppressive microenvironment to glioma progression. In patients with GB and mice with GB, the number of CD4 T lymphocytes in the blood (systemic lymphopenia) in some cases may correspond to the number of CD4 T lymphocytes in AIDS. In lymphoid organs, a deficiency of T-cells is seen, whereas in the bone marrow, many naive T-cells are found. This phenomenon is characteristic not only of brain tumors but also of other types of tumors, but this is relevant only when they grow intracerebral [45, 46]. Compared with tissue models of M2 GB and M6 GB, macrophage populations in a cell tumor model, GL261 mouse glioma, are low (on average 5.6%) [47]. According to other sources [48], this model is heavily infiltrated by immune cells. In humans, GB has variability in the content of immune cells. The expression of genes associated with the immune response, as well as the infiltration of macrophages, CD3+ and CD8+ T-cells increased during the initial phase of GB growth in mice and then decreased as tumor grew. This indicates a “correction” and “deprivation” of the immune response by the tumor [49].
The presence of CD3+ T-cells in GB samples indicates an immune response to the tumor. Treatment-induced release of tumor neoantigens are released, significantly increasing the CD3+ cell count. However, it has been suggested that during GB relapse, the CD3+ T-cell response may be limited due to a lack of antigens. According to some researchers, the presence of intratumoral CD3+ cells before treatment positively correlates with patient survival [50], but others have not confirmed this association [51]. Most human GB cases exhibit low CD3+ infiltration, consistent with the characteristic “cold” immune profile of the tumor. Approximately 25% of human GBs have moderate or high CD3+ infiltration [52], similar to the M6 GB model. Therefore, the M6 GB model represents a valuable tool for advancing immunotherapy strategies for this specific group of GB patients.
Compared with cell lines and chemically induced primary tumors, transplanted tumor tissue is more carcinogenic and exhibits more aggressive growth and rapid macrophage polarization to the M2 phenotype. Most immunosuppressive cytokines, enzymes, checkpoint ligands, cell surface molecules, and signal pathways are overexpressed in glioma stromal cells and macrophages/microglia, but not in tumor cells [49]. M6 GB and M2 GB are heavily infiltrated by tumor-associated lymphocytes and macrophages. This finding requires further study. Gene expression should aim to separately analyze gene expression in M2 GB and M6 GB tumor cells, as well as in the surrounding immune cells.
Mutations in the Hras, Pten, Pi3k, Mdm2, Tp63, Esr, Pgr,and Her2 genes, loss of Cdkn2a, Igf,and Akt, as well as MYC phosphorylation have been identified in DMBA-induced breast, blood, and skin tumor models [53–55]. Furthermore, these tumors are characterized by activation of Egfr and Mki-67, wild-type p53, low levels of mRNA and CDKN1A and PTEN proteins, and decreased expression and activity of the IDH1/2 enzyme, one of the main markers of gliomas [56].
Human GB is almost always classified as IDH-wildtype. It is characterized by activating alterations in the EGFR gene in approximately 57% of cases and in the TERT promoter in over 70–80% of cases. Notably, EGFR activation is less frequent in giant cell GB and gliosarcoma. Our study demonstrated that the M2 GB model, which contains a significant number of giant cells, exhibits consistent Egfr expression. In contrast to GB, IDH-mutant astrocytomas often harbor wild-type EGFR and PTEN genes, along with MGMT promoter methylation. These astrocytomas also frequently feature mutations in the TP53 and ATRX genes and the TERT promoter [57, 58]. Discrepancies between the MGMT promoter methylation status and treatment response in some patients may be attributed to a lack of correlation between MGMT methylation and actual MGMT protein expression levels in GB [59].
Aggressive tumor cells, especially poorly differentiated cells [60, 61], upregulate exocytosis via P-glycoprotein (P-gp) [62], which is expressed on the plasma membrane of endothelial cells of the blood-brain barrier. According to some researchers [63, 64], high expression of ABCB1 correlates with poor survival in GB patients. Others point to a correlation of longer survival in patients with high ABCB1 gene expression, though its independent prognostic value was not definitively established [65]. In pancreatic and kidney tumors, high expression of ABCB1 is a favorable prognostic factor. Increased expression of Abcb1 was found in M2 GB and M6 GB cells. The increased expression of P-gp is likely to be caused by prolonged carcinogen exposure, facilitating active efflux of DMBA from the cells [66]. P-gp expression is regulated by several signal pathways, including PI3K/Akt. According to this study, Pi3k is overexpressed in M2 GB. Alterations in the PI3K pathway were detected in 17% of patients with GB [67]. Impaired regulation of PI3K transforms tumors into more aggressive and recurrent diseases, and which is consistent with our morphological and clinical observations: exhibits more malignant histology and faster grow in M2 GB compared to M6 GB, which has a wild-type Pi3k expression.
Expression of the Tp53 gene, which encodes the p53 tumor suppressor and transcription factor, was elevated in the M2 GB model compared to intact brain tissue. In contrast, Tp53 expression was decreased in the M6 GB model. In M6 GB, the combination of low Tp53 expression and high Pi3k oncogene expression may drive the marked upregulation of Hif1a, Vegfa, Gdnf,and Egfr. Loss of p53 function contributes to oncogenesis in approximately 25–37% of GB. While inactivating TP53 mutations are common in the giant cell subtype of human GB, the M2 GB model — which also exhibits giant cell morphology — shows Tp53 overexpression, suggesting a distinct mechanism of p53 pathway dysregulation. Inactivating TP53 mutations are also prevalent in other CNS tumors, being found in over 50% of IDH1/2-mutant astrocytomas and large cell medulloblastomas.
For comparison, H3K27M-mutant diffuse midline gliomas are characterized by a high frequency of TP53 and IDH1/2 mutations, as well as PDGFRA amplification. These tumors typically retain wild-type ATRX, while TERT promoter mutations and EGFR amplification are rare [51].
Mutations in the TP53 gene, which lead to structural and functional alterations, are implicated in various tumor types [68]. For instance, missense TP53 mutations are characteristic of gliomas with IDH1/2 aberrations.
In our models, the Cdkn2a gene —a critical tumor suppressor, cell cycle regulator, and p53 pathway activator —was overexpressed in both M2 GB and M6 GB. This finding contrasts with the situation in human GB and WHO CNS Grade 4 astrocytoma, where homozygous deletion of CDKN2A is a hallmark alteration associated with high proliferative activity and poor prognosis [69].
The behavior of the p53 protein itself is complex. Exposure to carcinogens can initially increase p53 levels in non-tumor cells [70]. However, prion-like aggregation of mutant p53 can lead to its functional inactivation; these aggregates, including oligomers and amyloid-like fibrils, can sequester wild-type p53, promoting oncogenesis [71, 72]. p53 levels also critically influence cell fate: low levels facilitate stem cell formation and proliferation, while high levels promote differentiation [73]. Despite the high Tp53 expression in M2 GB, the Sox2 gene (a stemness marker) was also highly expressed in both models. This suggests that M6 GB tumors may be more differentiated than M2 GB, potentially due to differences in p53 activity beyond mere expression levels. Furthermore, p53 can interact with mitochondrial peptidylprolyl isomerase F (PPIF) to induce necrotic cell death [74]. This mechanism is consistent with our morphological observations, where necrosis was more pronounced and extensive in M2 GB than in M6 GB.
M2 GB and M6 GB tissues exhibited increased expression of the Sox2 gene, a key transcription factor for stem cell maintenance and a marker of undifferentiated cells. SOX2 overexpression is a hallmark of various poorly differentiated tumors, including GB [75]. The role of SOX2 in tumor progression appears context-dependent. It has been demonstrated to promote cell migration and confer resistance to chemotherapy [76]. Conversely, some studies indicate that its suppression can also be associated with tumor invasion and treatment resistance, highlighting the complexity of its function. SOX2 is highly expressed in the developing central nervous system and is essential for neural stem cell function [77]. Gliomas, similar to neural stem cells, exhibit an accessible chromatin state at the SOX2 enhancer cluster, which contributes to its sustained high expression and drives tumor cell proliferation [78].
In contrast to the frequent PTEN deficiency observed in human GB, both M2 GB and M6 GB mouse models exhibited Pten overexpression. The functional consequences of PTEN overexpression appear to be context-dependent. While some studies demonstrate that it can induce apoptosis, disrupt mitochondrial function, and sensitize glioma cells to chemotherapy, others report that it may promote tumor cell motility and dedifferentiation in certain contexts [79–85]. The latter is supported by our finding of elevated Cd44 and Sox2 expression in the M6 GB model, markers associated with a less differentiated, aggressive state.
This paradox aligns with a broader concept in oncology: while tumor suppressor overexpression is typically intended to halt growth and promote differentiation or apoptosis [66], it can, under specific conditions, paradoxically drive tumor progression. Although carcinogenesis is primarily driven by the loss of tumor suppressors and activation of oncogenes, sustained overexpression of certain tumor suppressors has been linked to the progression of colorectal, breast, ovarian, head and neck cancers, and lymphomas, correlating with reduced patient survival [82, 83]. In these specific contexts, such genes may exhibit oncogenic properties.
PTEN is a known negative regulator of the PI3K/AKT/mTOR signaling cascade, a key pathway promoting tumor cell growth. Paradoxically, both M2 GB and M6 GB models exhibited concurrent Pten overexpression and Pi3k activation. This contrasts with the situation in melanoma, where PTEN loss is common and associated with reduced T-cell infiltration and therapy resistance, making PI3K pathway inhibition a rational therapeutic strategy [84]. In our study, however, Pten overexpression in the M2 GB and M6 GB models was associated with substantial tumor infiltration by T-cells.
Expression of the Mki67 gene, encoding the Ki-67 proliferation marker, was elevated in both M2 GB and M6 GB cells, consistent with the observed high mitotic activity. From a therapeutic perspective, promoter methylation of the MGMT gene, which silences this DNA repair enzyme, is a favorable prognostic marker in GB patients treated with alkylating agents [57]. In M2 GB and M6 GB models, Mgmt expression remained unchanged, suggesting intrinsic resistance to such chemotherapy. This makes these models particularly valuable for studying mechanisms of chemoresistance and developing novel antitumor strategies to overcome it.
The VEGFA and HIF1α genes are overexpressed in many human tumors, a pattern recapitulated in the M2 GB and M6 GB mouse models. VEGF-A is a key regulator of angiogenesis, controlling endothelial cell proliferation and vascular permeability; however, its function can be context-dependent, exhibiting both pro- and anti-angiogenic effects [85]. HIF1α is a well-established prognostic marker for predicting tumor response to treatment. In tumors, receptors for various growth factors are often constitutively active, sustaining downstream signaling even in the presence of low ligand concentrations. This dysregulated activation of receptor tyrosine kinase pathways is a major driver of tumor growth, which may represent an aberrant attempt by cancer cells to overcome stress signals that would otherwise trigger differentiation or cell death [66].
The orthologue of the GDNF gene, which encodes a dopaminergic neurotrophic factor and a ligand for the TGF-β superfamily, was overexpressed in M6 GB. GDNF can activate the SMAD transcription factor pathway, influencing cell cycle progression [86].
CD133 (prominin-1) is a marker of tumor-initiating cells in various solid tumors. Expression of its ortholog was significantly elevated in the M2 GB model. A high frequency of CD133+ cells is generally associated with resistance to chemo- and radiotherapy and poor patient survival [87]. However, the biology of CD133 is complex, as some differentiated cells can express it, and CD133-negative cell populations can also initiate tumors [88].
Pdgfra levels were increased in M2 GB. PDGFRA activation leads to phosphorylation of PIK3R1, triggering downstream signaling cascades including calcium mobilization and activation of PKC, AKT1, HRAS/MAPK/ERK, and STAT pathways, thereby promoting tumor growth and survival [89].
The S100B protein, a neurotrophic factor and one of the most abundant soluble proteins in the brain [90], was overexpressed in both the M2 GB and M6 GB models. S100B promotes astrocytosis and axonal growth. It exhibits a higher binding affinity for Zn2+ than for Ca2+. In glioma patients, a high serum level of S100B serves as a prognostic marker, and the S100B protein family is known to regulate glioma stem cells and mediate epithelial-mesenchymal transition in GB [91].
It is important to note that all the studied genes encode multiple protein isoforms. Mechanisms such as alternative splicing and the use of alternative promoters increase transcript diversity, leading to a variety of protein isoforms with potentially different functions [92, 93]. Furthermore, gene activity is modulated by epigenetic mechanisms and post-translational modifications. Critically, even elevated mRNA expression in tumor cells may not result in increased protein levels due to post-transcriptional repression [94, 95]. Therefore, future studies should aim to characterize the specific protein isoforms expressed and quantify their levels to fully understand their functional role in these models.
Therefore, the carcinogen DMBA induces oncogenesis in brain tissue through the formation of DNA adducts and dysregulation of genes governing key cellular processes, including angiogenesis, proliferation, invasion, development, transcription, apoptosis, DNA repair, cytoskeleton organization, metabolism, and intercellular signaling [96]. This leads to genomic instability and tumor progression. Sequential transplantation of DMBA-induced tumor tissue, along with associated tumor-associated macrophages and other microenvironmental factors, can further reshape the tumor’s genetic landscape and drive malignant progression.
Gene expression profiling of the M2 GB and M2 GB strains revealed aberrant activation of the tumor suppressors Pten, Cdkn2a, and Tp53 in M2 GB. These alterations indicate that the compensatory cellular response to DMBA exposure — aimed at restoring DNA integrity, suppressing proliferation, facilitating carcinogen removal, and eliminating abnormal cells — was ultimately unsuccessful. The initial stages of DMBA-induced oncogenesis are characterized by non-proliferative changes, inflammation, and capsule formation [97], representing a host defense mechanism to isolate the carcinogen. Tumor cells, despite their impaired self-regulation, retain fundamental genomic regulatory features of healthy cells. Many of the genetic modifications observed in tumors may represent an adaptive response to carcinogenic stress and an attempt to compensate for defects in DNA replication [66].
The use of intracerebral tissue models like M2 GB and M6 GB in immunocompetent mice provides a valuable platform for generating new data on tumor biology, diagnosis, and therapy. These models, along with other established transplanted models such as the GB 101.8 rat glioma, play a major role in developing diagnostic tools [98–100], advancing therapeutic strategies [101], and elucidating the mechanisms of carcinogenesis [102, 103].
A primary limitation of this study is its small sample size.
Conclusion
Experimental M2 GB and M6 GB tissue models recapitulate key features of human gliomagenesis based on clinical features of this disease (cachexia, metabolic disturbances, tumor-associated coagulopathy) and morphology (invasive aggressive growth, nuclear and cellular polymorphism, high mitotic activity, pronounced vascularization, and necrosis). Substantial immune cell infiltration (comprising up to 50% of the tumor tissue), coupled with altered expression of genes central to oncogenesis, establishes these models as highly suitable for investigating carcinogenesis and evaluating novel antitumor therapies.
Gene expression profiling revealed distinct transcriptional programs for each model. A common signature of upregulated genes in both M2 GB and M6 GB included tumor suppressors (Cdkn2a, Pten), the cellular transporter (Abcb1), the proliferation marker (Mki67), transcription factors (Hif1a, Sox2), as well as neurotrophic (S100b) and angiogenic (Vegfa) factors. Model-specific profiles were also identified: M6 GB showed unique upregulation of the growth factor receptor Pdgfra, the differentiation marker Cd133, and the tumor suppressor Tp53. In contrast, M2 GB was characterized by elevated expression of the signaling factor Pi3k, the glial filament protein Gfap, and the growth factor Gdnf.
Furthermore, comparative analysis showed that M6 GB had higher mRNA levels of genes involved in cell adhesion (Cd44), proliferation (Pi3k, Hif1a, Gdnf), and growth signaling (Egfr) compared to M2 GB. Conversely, M6 GB exhibited lower expression of genes related to differentiation, proliferation, and apoptosis —Cdkn2a, Tp53, and Cd133 —as well as the Pdgfra receptor, relative to M2 GB.
In conclusion, the M2 GB and M6 GB models, with their human-like intratumoral immune landscape, clinical and morphological features, and distinct gene expression patterns, represent valuable tools for advancing research in gliomagenesis and for developing innovative diagnostic and therapeutic strategies.
Funding. The study was supported by the Ministry of Education and Science of the Russian Federation under State Contract No.123030700107-4.
Conflict of interest. The authors declare no conflicts of interest.
References
- Wu W., Klockow J.L., Zhang M., Lafortune F., Chang E., Jin L., Wu Y., Daldrup-Link H.E. Glioblastoma multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol Res 2021; 171: 105780, https://doi.org/10.1016/j.phrs.2021.105780.
- Background lesions in laboratory animals. A color atlas. McInnes E.F., Mann P. (editors). Elsevier Ltd.; 2011, https://doi.org/10.1016/C2009-0-41283-2.
- Lampreht Tratar U., Horvat S., Cemazar M. Transgenic mouse models in cancer research. Front Oncol 2018; 8: 268, https://doi.org/10.3389/fonc.2018.00268.
- Ireson C.R., Alavijeh M.S., Palmer A.M., Fowler E.R., Jones H.J. The role of mouse tumour models in the discovery and development of anticancer drugs. Br J Cancer 2019; 121(2): 101–108, https://doi.org/10.1038/s41416-019-0495-5.
- Sahu U., Barth R.F., Otani Y., McCormack R., Kaur B. Rat and mouse brain tumor models for experimental neuro-oncology research. J Neuropathol Exp Neurol 2022; 81(5): 312–329, https://doi.org/10.1093/jnen/nlac021.
- Haddad A.F., Young J.S., Amara D., Berger M.S., Raleigh D.R., Aghi M.K., Butowski N.A. Mouse models of glioblastoma for the evaluation of novel therapeutic strategies. Neurooncol Adv 2021; 3(1): vdab100, https://doi.org/10.1093/noajnl/vdab100.
- Lowenstein P.R., Castro M.G. Uncertainty in the translation of preclinical experiments to clinical trials. Why do most phase III clinical trials fail? Curr Gene Ther 2009; 9(5): 368–374, https://doi.org/10.2174/156652309789753392.
- Fogel D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp Clin Trials Commun 2018; 11: 156–164, https://doi.org/10.1016/j.conctc.2018.08.001.
- Mirzayans R., Murray D. What are the reasons for continuing failures in cancer therapy? Are misleading/inappropriate preclinical assays to be blamed? Might some modern therapies cause more harm than benefit? Int J Mol Sci 2022; 23(21): 13217, https://doi.org/10.3390/ijms232113217.
- Arutyunyan I.V., Soboleva A.G., Kovtunov E.A., Kosyreva A.M., Kudelkina V.V., Alekseeva A.I., Elchaninov A.V., Jumaniyazova E.D., Goldshtein D.V., Bolshakova G.B., Fatkhudinov T.K. Gene expression profile of 3D spheroids in comparison with 2D cell cultures and tissue strains of diffuse high-grade gliomas. Bull Exp Biol Med 2023; 175(4): 576–584, https://doi.org/10.1007/s10517-023-05906-y.
- Alekseeva A., Drozd S., Nikitin P., Postnov A., Lipengolts A., Skribitsky V., Finogenova Y., Shpakova K., Khalansky A., Pronin I., Pavlova G. Comparative morphological and molecular genetic characteristics of experimental glioblastoma 101/8 and C6. In: Neuroscience for medicine and psychology. Sudak; 2023; p. 34–35, https://doi.org/10.29003/m3154.sudak.ns2023-19/34-35.
- Paster E.V., Villines K.A., Hickman D.L. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med 2009; 59(3): 234–241.
- Fedoseeva V.V., Khalansky A.S., Mkhitarov V.A., Tsvetkov I.S., Malinovskaya Y.A., Maksimenko O.O., Gelperina S.E., Balabanyan V.Y., Razzhivina V.A., Gorelikov P.L., Mikhailova L.P., Makarova O.V. Anti-tumor activity of doxorubicin-loaded poly(lactide-co-glycolide) nanoparticles in the experimental glioblastoma. Klinicheskaya i eksperimental’naya morfologiya 2017; 2(22): 65–71.
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29(9): e45, https://doi.org/10.1093/nar/29.9.e45.
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3(7): RESEARCH0034, https://doi.org/10.1186/gb-2002-3-7-research0034.
- Inoue A., Ohnishi T., Nishikawa M., Watanabe H., Kusakabe K., Taniwaki M., Yano H., Ohtsuka Y., Matsumoto S., Suehiro S., Yamashita D., Shigekawa S., Takahashi H., Kitazawa R., Tanaka J., Kunieda T. Identification of CD44 as a reliable biomarker for glioblastoma invasion: based on magnetic resonance imaging and spectroscopic analysis of 5-aminolevulinic acid fluorescence. Biomedicines 2023; 11(9): 2369, https://doi.org/10.3390/biomedicines11092369.
- Wang L.J., Lv P., Lou Y. Alarm signal S100-related signature is correlated with tumor microenvironment and predicts prognosis in glioma. Dis Markers 2022; 2022: 4968555, https://doi.org/10.1155/2022/4968555.
- Yuan Q., Zuo F.X., Cai H.Q., Qian H.P., Wan J.H. Identifying differential expression genes and prognostic signature based on subventricular zone involved glioblastoma. Front Genet 2022; 13: 912227, https://doi.org/10.3389/fgene.2022.912227.
- Luo X., Xu S., Zhong Y., Tu T., Xu Y., Li X., Wang B., Yang F. High gene expression levels of VEGFA and CXCL8 in the peritumoral brain zone are associated with the recurrence of glioblastoma: a bioinformatics analysis. Oncol Lett 2019; 18(6): 6171–6179, https://doi.org/10.3892/ol.2019.10988.
- Liu W., Lv G., Li Y., Li L., Wang B. Downregulation of CDKN2A and suppression of cyclin D1 gene expressions in malignant gliomas. J Exp Clin Cancer Res 2011; 30(1): 76, https://doi.org/10.1186/1756-9966-30-76.
- Shiraishi S., Tada K., Nakamura H., Makino K., Kochi M., Saya H., Kuratsu J., Ushio Y. Influence of p53 mutations on prognosis of patients with glioblastoma. Cancer 2002; 95(2): 249–257, https://doi.org/10.1002/cncr.10677.
- Dahlrot R.H., Bangsø J.A., Petersen J.K., Rosager A.M., Sørensen M.D., Reifenberger G., Hansen S., Kristensen B.W. Prognostic role of Ki-67 in glioblastomas excluding contribution from non-neoplastic cells. Sci Rep 2021; 11(1): 17918, https://doi.org/10.1038/s41598-021-95958-9.
- Hashemi M., Etemad S., Rezaei S., Ziaolhagh S., Rajabi R., Rahmanian P., Abdi S., Koohpar Z.K., Rafiei R., Raei B., Ahmadi F., Salimimoghadam S., Aref A.R., Zandieh M.A., Entezari M., Taheriazam A., Hushmandi K. Progress in targeting PTEN/PI3K/Akt axis in glioblastoma therapy: revisiting molecular interactions. Biomed Pharmacother 2023; 158: 114204, https://doi.org/10.1016/j.biopha.2022.114204.
- Sfifou F., Hakkou E.M., Bouaiti E.A., Slaoui M., Errihani H., Al Bouzidi A., Abouqal R., El Ouahabi A., Cherradi N. Correlation of immunohistochemical expression of HIF-1alpha and IDH1 with clinicopathological and therapeutic data of moroccan glioblastoma and survival analysis. Ann Med Surg (Lond) 2021; 69: 102731, https://doi.org/10.1016/j.amsu.2021.102731.
- Yu W., Ren X., Hu C., Tan Y., Shui Y., Chen Z., Zhang L., Peng J., Wei Q. Glioma SOX2 expression decreased after adjuvant therapy. BMC Cancer 2019; 19(1): 1087, https://doi.org/10.1186/s12885-019-6292-y.
- Abdoli Shadbad M., Nejadi Orang F., Baradaran B. CD133 significance in glioblastoma development: in silico and in vitro study. Eur J Med Res 2024; 29(1): 154, https://doi.org/10.1186/s40001-024-01754-2.
- Farsi Z., Allahyari Fard N. The identification of key genes and pathways in glioblastoma by bioinformatics analysis. Mol Cell Oncol 2023; 10(1): 2246657, https://doi.org/10.1080/23723556.2023.2246657.
- Yu Z., Li H., Wang M., Luo W., Xue Y. GDNF regulates lipid metabolism and glioma growth through RET/ERK/HIF-1/SREBP-1. Int J Oncol 2022; 61(3): 109, https://doi.org/10.3892/ijo.2022.5399.
- Szylberg M., Sokal P., Śledzińska P., Bebyn M., Krajewski S., Szylberg Ł., Szylberg A., Szylberg T., Krystkiewicz K., Birski M., Harat M., Włodarski R., Furtak J. MGMT promoter methylation as a prognostic factor in primary glioblastoma: a single-institution observational study. Biomedicines 2022, https://doi.org/10.3390/biomedicines10082030.
- Roy L.O., Lemelin M., Blanchette M., Poirier M.B., Aldakhil S., Fortin D. Expression of ABCB1, ABCC1 and 3 and ABCG2 in glioblastoma and their relevance in relation to clinical survival surrogates. J Neurooncol 2022; 160(3): 601–609, https://doi.org/10.1007/s11060-022-04179-1.
- Ahmadipour Y., Gembruch O., Pierscianek D., Sure U., Jabbarli R. Does the expression of glial fibrillary acid protein (GFAP) stain in glioblastoma tissue have a prognostic impact on survival? Neurochirurgie 2020; 66(3): 150–154, https://doi.org/10.1016/j.neuchi.2019.12.012.
- Dhawan A., Manem V.S.K., Yeaney G., Lathia J.D., Ahluwalia M.S. EGFR pathway expression persists in recurrent glioblastoma independent of amplification status. Cancers (Basel) 2023; 15(3): 670, https://doi.org/10.3390/cancers15030670.
- Goldman O., Adler L.N., Hajaj E., Croese T., Darzi N., Galai S., Tishler H., Ariav Y., Lavie D., Fellus-Alyagor L., Oren R., Kuznetsov Y., David E., Jaschek R., Stossel C., Singer O., Malitsky S., Barak R., Seger R., Erez N., Amit I., Tanay A., Saada A., Golan T., Rubinek T., Sang Lee J., Ben-Shachar S., Wolf I., Erez A. Early Infiltration of innate immune cells to the liver depletes HNF4α and promotes extrahepatic carcinogenesis. Cancer Discov 2023; 13(7): 1616–1635, https://doi.org/10.1158/2159-8290.CD-22-1062.
- Law M.L. Cancer cachexia: pathophysiology and association with cancer-related pain. Front Pain Res (Lausanne) 2022; 3: 971295, https://doi.org/10.3389/fpain.2022.971295.
- Olson B., Diba P., Korzun T., Marks D.L. Neural mechanisms of cancer cachexia. Cancers (Basel) 2021; 13(16): 3990, https://doi.org/10.3390/cancers13163990.
- Zhong W., Jina H., Rathore P., Wong E.L., Mancuso P., Lalak N., Hayden L., Haghighi K. A case report of priapism with unusual presentation and clinical course. Urol Case Rep 2017; 12: 70–72, https://doi.org/10.1016/j.eucr.2017.03.009.
- Shelton L.M., Mukherjee P., Huysentruyt L.C., Urits I., Rosenberg J.A., Seyfried T.N. A novel pre-clinical in vivo mouse model for malignant brain tumor growth and invasion. J Neurooncol 2010; 99(2): 165–176, https://doi.org/10.1007/s11060-010-0115-y.
- Cui P., Shao W., Huang C., Wu C.J., Jiang B., Lin D. Metabolic derangements of skeletal muscle from a murine model of glioma cachexia. Skelet Muscle 2019; 9(1): 3, https://doi.org/10.1186/s13395-018-0188-4.
- Monteiro R.Q., Lima L.G., Gonçalves N.P., De Souza M.R., Leal A.C., Demasi M.A., Sogayar M.C., Carneiro-Lobo T.C. Hypoxia regulates the expression of tissue factor pathway signaling elements in a rat glioma model. Oncol Lett 2016; 12(1): 315–322, https://doi.org/10.3892/ol.2016.4593.
- Onizuka H., Masui K., Komori T. Diffuse gliomas to date and beyond 2016 WHO Classification of Tumours of the central nervous system. Int J Clin Oncol 2020; 25(6): 997–1003, https://doi.org/10.1007/s10147-020-01695-w.
- Himes B.T., Geiger P.A., Ayasoufi K., Bhargav A.G., Brown D.A., Parney I.F. Immunosuppression in glioblastoma: current understanding and therapeutic implications. Front Oncol 2021; 11: 770561, https://doi.org/10.3389/fonc.2021.770561.
- Alghamri M.S., McClellan B.L., Hartlage C.S., Haase S., Faisal S.M., Thalla R., Dabaja A., Banerjee K., Carney S.V., Mujeeb A.A., Olin M.R., Moon J.J., Schwendeman A., Lowenstein P.R., Castro M.G. Targeting neuroinflammation in brain cancer: uncovering mechanisms, pharmacological targets, and neuropharmaceutical developments. Front Pharmacol 2021; 12: 680021, https://doi.org/10.3389/fphar.2021.680021.
- Andersen J.K., Miletic H., Hossain J.A. Tumor-associated macrophages in gliomas-basic insights and treatment opportunities. Cancers (Basel) 2022; 14(5): 1319, https://doi.org/10.3390/cancers14051319.
- Georgieva P.B., Mathivet T., Alt S., Giese W., Riva M., Balcer M., Gerhardt H. Long-lived tumor-associated macrophages in glioma. Neurooncol Adv 2020; 2(1): vdaa127, https://doi.org/10.1093/noajnl/vdaa127.
- Rodrigues L.F., Camacho A.H.D.S., Spohr T.C.L.S.E. Secondary glioblastoma metastasis outside the central nervous system in a young HIV-infected patient. Ther Adv Med Oncol 2020; 12: 1758835920923432, https://doi.org/10.1177/1758835920923432.
- Chongsathidkiet P., Jackson C., Koyama S., Loebel F., Cui X., Farber S.H., Woroniecka K., Elsamadicy A.A., Dechant C.A., Kemeny H.R., Sanchez-Perez L., Cheema T.A., Souders N.C., Herndon J.E., Coumans J.V., Everitt J.I., Nahed B.V., Sampson J.H., Gunn M.D., Martuza R.L., Dranoff G., Curry W.T., Fecci P.E. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med 2018; 24(9): 1459–1468, https://doi.org/10.1038/s41591-018-0135-2.
- Sanchez V.E., Lynes J.P., Walbridge S., Wang X., Edwards N.A., Nwankwo A.K., Sur H.P., Dominah G.A., Obungu A., Adamstein N., Dagur P.K., Maric D., Munasinghe J., Heiss J.D., Nduom E.K. GL261 luciferase-expressing cells elicit an anti-tumor immune response: an evaluation of murine glioma models. Sci Rep 2020; 10(1): 11003, https://doi.org/10.1038/s41598-020-67411-w.
- Brodin P., Davis M.M. Human immune system variation. Nat Rev Immunol 2017; 17(1): 21–29, https://doi.org/10.1038/nri.2016.125.
- Maire C.L., Mohme M., Bockmayr M., Fita K.D., Riecken K., Börnigen D., Alawi M., Failla A., Kolbe K., Zapf S., Holz M., Neumann K., Dührsen L., Lange T., Fehse B., Westphal M., Lamszus K. Glioma escape signature and clonal development under immune pressure. J Clin Invest 2020; 130(10): 5257–5271, https://doi.org/10.1172/JCI138760.
- Brown C.E., Hibbard J.C., Alizadeh D., Blanchard M.S., Natri H.M., Wang D., Ostberg J.R., Aguilar B., Wagner J.R., Paul J.A., Starr R., Wong R.A., Chen W., Shulkin N., Aftabizadeh M., Filippov A., Chaudhry A., Ressler J.A., Kilpatrick J., Myers-McNamara P., Chen M., Wang L.D., Rockne R.C., Georges J., Portnow J., Barish M.E., D’Apuzzo M., Banovich N.E., Forman S.J., Badie B. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial. Nat Med 2024; 30(4): 1001–1012, https://doi.org/10.1038/s41591-024-02875-1.
- Sobhani N., Bouchè V., Aldegheri G., Rocca A., D’Angelo A., Giudici F., Bottin C., Donofrio C.A., Pinamonti M., Ferrari B., Panni S., Cominetti M., Aliaga J., Ungari M., Fioravanti A., Zanconati F., Generali D. Analysis of PD-L1 and CD3 expression in glioblastoma patients and correlation with outcome: a single center report. Biomedicines 2023; 11(2): 311, https://doi.org/10.3390/biomedicines11020311.
- Musca B., Russo M.G., Tushe A., Magri S., Battaggia G., Pinton L., Bonaudo C., Della Puppa A., Mandruzzato S. The immune cell landscape of glioblastoma patients highlights a myeloid-enriched and immune suppressed microenvironment compared to metastatic brain tumors. Front Immunol 2023; 14: 1236824, https://doi.org/10.3389/fimmu.2023.1236824.
- Nasti T.H., Cochran J.B., Tsuruta Y., Yusuf N., McKay K.M., Athar M., Timares L., Elmets C.A. A murine model for the development of melanocytic nevi and their progression to melanoma. Mol Carcinog 2016; 55(5): 646–658, https://doi.org/10.1002/mc.22310.
- Todorova V.K., Kaufmann Y., Luo S., Klimberg V.S. Modulation of p53 and c-myc in DMBA-induced mammary tumors by oral glutamine. Nutr Cancer 2006; 54(2): 263–273, https://doi.org/10.1207/s15327914nc5402_13.
- Fidianingsih I., Aryandono T., Widyarini S., Herwiyanti S. Profile of histopathological type and molecular subtypes of mammary cancer of DMBA-induced rat and its relevancy to human breast cancer. J Med Sci 2022; 10(A): 71–78, https://doi.org/10.3889/oamjms.2022.7975.
- Robbins D., Wittwer J.A., Codarin S., Circu M.L., Aw T.Y., Huang T.T., Van Remmen H., Richardson A., Wang D.B., Witt S.N., Klein R.L., Zhao Y. Isocitrate dehydrogenase 1 is downregulated during early skin tumorigenesis which can be inhibited by overexpression of manganese superoxide dismutase. Cancer Sci 2012; 103(8): 1429–1433, https://doi.org/10.1111/j.1349-7006.2012.02317.x.
- Evidence based practice in neuro-oncology. Mallick S., Giridhar P., Rath G.K. (editors). Springer Singapore; 2021, https://doi.org/10.1007/978-981-16-2659-3.
- Marker D.F., Agnihotri S., Amankulor N., Murdoch G.H., Pearce T.M. The dominant TP53 hotspot mutation in IDH -mutant astrocytoma, R273C, has distinctive pathologic features and sex-specific prognostic implications. Neurooncol Adv 2021; 4(1): vdab182, https://doi.org/10.1093/noajnl/vdab182.
- Butler M., Pongor L., Su Y.T., Xi L., Raffeld M., Quezado M., Trepel J., Aldape K., Pommier Y., Wu J. MGMT status as a clinical biomarker in glioblastoma. Trends Cancer 2020; 6(5): 380–391, https://doi.org/10.1016/j.trecan.2020.02.010.
- Ohtsuki S., Kamoi M., Watanabe Y., Suzuki H., Hori S., Terasaki T. Correlation of induction of ATP binding cassette transporter A5 (ABCA5) and ABCB1 mRNAs with differentiation state of human colon tumor. Biol Pharm Bull 2007; 30(6): 1144–1146, https://doi.org/10.1248/bpb.30.1144.
- Oda Y., Saito T., Tateishi N., Ohishi Y., Tamiya S., Yamamoto H., Yokoyama R., Uchiumi T., Iwamoto Y., Kuwano M., Tsuneyoshi M. ATP-binding cassette superfamily transporter gene expression in human soft tissue sarcomas. Int J Cancer 2005; 114(6): 854–862, https://doi.org/10.1002/ijc.20589.
- Ahmed S., Khan H., Aschner M., Mirzae H., Küpeli Akkol E., Capasso R. Anticancer potential of furanocoumarins: mechanistic and therapeutic aspects. Int J Mol Sci 2020; 21(16): 5622, https://doi.org/10.3390/ijms21165622.
- Robey R.W., Massey P.R., Amiri-Kordestani L., Bates S.E. ABC transporters: unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med Chem 2010; 10(8): 625–633, https://doi.org/10.2174/187152010794473957.
- Amiri-Kordestani L., Basseville A., Kurdziel K., Fojo A.T., Bates S.E. Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updat 2012; 15(1-2): 50–61, https://doi.org/10.1016/j.drup.2012.02.002.
- Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A., Odeberg J., Djureinovic D., Takanen J.O., Hober S., Alm T., Edqvist P.H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J.M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F. Tissue-based map of the human proteome. Science 2015; 347(6220): 1260419, https://doi.org/10.1126/science.1260419.
- Suba Z. Rosetta stone for cancer cure: comparison of the anticancer capacity of endogenous estrogens, synthetic estrogens and antiestrogens. Oncol Rev 2023; 17: 10708, https://doi.org/10.3389/or.2023.10708.
- Padovan M., Maccari M., Bosio A., De Toni C., Vizzaccaro S., Cestonaro I., Corrà M., Caccese M., Cerretti G., Zagonel V., Lombardi G. Actionable molecular alterations in newly diagnosed and recurrent IDH1/2 wild-type glioblastoma patients and therapeutic implications: a large mono-institutional experience using extensive next-generation sequencing analysis. Eur J Cancer 2023; 191: 112959, https://doi.org/10.1016/j.ejca.2023.112959.
- Huszno J., Grzybowska E. TP53 mutations and SNPs as prognostic and predictive factors in patients with breast cancer. Oncol Lett 2018; 16(1): 34–40, https://doi.org/10.3892/ol.2018.8627.
- Robertson L.B., Armstrong G.N., Olver B.D., Lloyd A.L., Shete S., Lau C., Claus E.B., Barnholtz-Sloan J., Lai R., Il’yasova D., Schildkraut J., Bernstein J.L., Olson S.H., Jenkins R.B., Yang P., Rynearson A.L., Wrensch M., McCoy L., Wienkce J.K., McCarthy B., Davis F., Vick N.A., Johansen C., Bødtcher H., Sadetzki S., Bruchim R.B., Yechezkel G.H., Andersson U., Melin B.S., Bondy M.L., Houlston R.S. Survey of familial glioma and role of germline p16INK4A/p14ARF and p53 mutation. Fam Cancer 2010; 9(3): 413–421, https://doi.org/10.1007/s10689-010-9346-5.
- Maltzman W., Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol 1984; 4(9): 1689–1694, https://doi.org/10.1128/mcb.4.9.1689-1694.1984.
- Park S.K., Park S., Pentek C., Liebman S.W. Tumor suppressor protein p53 expressed in yeast can remain diffuse, form a prion, or form unstable liquid-like droplets. iScience 2020; 24(1): 102000, https://doi.org/10.1016/j.isci.2020.102000.
- Navalkar A., Ghosh S., Pandey S., Paul A., Datta D., Maji S.K. Prion-like p53 amyloids in cancer. Biochemistry 2020; 59(2): 146–155, https://doi.org/10.1021/acs.biochem.9b00796.
- Levine A.J., Puzio-Kuter A.M., Chan C.S., Hainaut P. The role of the p53 protein in stem-cell biology and epigenetic regulation. Cold Spring Harb Perspect Med 2016; 6(9): a026153, https://doi.org/10.1101/cshperspect.a026153.
- Hill K.A., Sommer S.S. p53 as a mutagen test in breast cancer. Environ Mol Mutagen 2002; 39(2–3): 216–227, https://doi.org/10.1002/em.10065.
- Mirzaei S., Paskeh M.D.A., Entezari M., Mirmazloomi S.R., Hassanpoor A., Aboutalebi M., Rezaei S., Hejazi E.S., Kakavand A., Heidari H., Salimimoghadam S., Taheriazam A., Hashemi M., Samarghandian S. SOX2 function in cancers: association with growth, invasion, stemness and therapy response. Biomed Pharmacother 2022; 156: 113860, https://doi.org/10.1016/j.biopha.2022.113860.
- Zhu Y., Huang S., Chen S., Chen J., Wang Z., Wang Y., Zheng H. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis 2021; 12(5): 449, https://doi.org/10.1038/s41419-021-03733-5.
- Zhang S., Xiong X., Sun Y. Functional characterization of SOX2 as an anticancer target. Signal Transduct Target Ther 2020; 5(1): 135, https://doi.org/10.1038/s41392-020-00242-3.
- Abatti L.E., Lado-Fernández P., Huynh L., Collado M., Hoffman M.M., Mitchell J.A. Epigenetic reprogramming of a distal developmental enhancer cluster drives SOX2 overexpression in breast and lung adenocarcinoma. Nucleic Acids Res 2023; 51(19): 10109–10131, https://doi.org/10.1093/nar/gkad734.
- Bao L., Li X., Lin Z. PTEN overexpression promotes glioblastoma death through triggering mitochondrial division and inactivating the Akt pathway. J Recept Signal Transduct Res 2019; 39(3): 215–225, https://doi.org/10.1080/10799893.2019.1655051.
- Yokoi A., Minami M., Hashimura M., Oguri Y., Matsumoto T., Hasegawa Y., Nakagawa M., Ishibashi Y., Ito T., Ohhigata K., Harada Y., Fukagawa N., Saegusa M. PTEN overexpression and nuclear β-catenin stabilization promote morular differentiation through induction of epithelial-mesenchymal transition and cancer stem cell-like properties in endometrial carcinoma. Cell Commun Signal 2022; 20(1): 181, https://doi.org/10.1186/s12964-022-00999-w.
- Li B., Zhang J., Su Y., Hou Y., Wang Z., Zhao L., Sun S., Fu H. Overexpression of PTEN may increase the effect of pemetrexed on A549 cells via inhibition of the PI3K/AKT/mTOR pathway and carbohydrate metabolism. Mol Med Rep 2019; 20(4): 3793–3801, https://doi.org/10.3892/mmr.2019.10617.
- Romagosa C., Simonetti S., López-Vicente L., Mazo A., Lleonart M.E., Castellvi J., Ramon y Cajal S. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011; 30(18): 2087–2097, https://doi.org/10.1038/onc.2010.614.
- Yehia L., Keel E., Eng C. The clinical spectrum of PTEN mutations. Annu Rev Med 2020; 71: 103–116, https://doi.org/10.1146/annurev-med-052218-125823.
- Cabrita R., Mitra S., Sanna A., Ekedahl H., Lövgren K., Olsson H., Ingvar C., Isaksson K., Lauss M., Carneiro A., Jönsson G. The role of PTEN loss in immune escape, melanoma prognosis and therapy response. Cancers (Basel) 2020; 12(3): 742, https://doi.org/10.3390/cancers12030742.
- Pentheroudakis G., Mavroeidis L., Papadopoulou K., Koliou G.A., Bamia C., Chatzopoulos K., Samantas E., Mauri D., Efstratiou I., Pectasides D., Makatsoris T., Bafaloukos D., Papakostas P., Papatsibas G., Bombolaki I., Chrisafi S., Kourea H.P., Petraki K., Kafiri G., Fountzilas G., Kotoula V. Angiogenic and antiangiogenic VEGFA splice variants in colorectal cancer: prospective retrospective cohort study in patients treated with irinotecan-based chemotherapy and bevacizumab. Clin Colorectal Cancer 2019; 18(4): e370–e384, https://doi.org/10.1016/j.clcc.2019.07.007.
- 86GDNF glial cell derived neurotrophic factor. URL: https://www.ncbi.nlm.nih.gov/gene/2668.
- Vora P., Venugopal C., Salim S.K., Tatari N., Bakhshinyan D., Singh M., Seyfrid M., Upreti D., Rentas S., Wong N., Williams R., Qazi M.A., Chokshi C., Ding A., Subapanditha M., Savage N., Mahendram S., Ford E., Adile A.A., McKenna D., McFarlane N., Huynh V., Wylie R.G., Pan J., Bramson J., Hope K., Moffat J., Singh S. The rational development of CD133-targeting immunotherapies for glioblastoma. Cell Stem Cell 2020; 26(6): 832–844.e6, https://doi.org/10.1016/j.stem.2020.04.008.
- Irollo E., Pirozzi G. CD133: to be or not to be, is this the real question? Am J Transl Res 2013; 5(6): 563–581.
- Razmara M., Heldin C.H., Lennartsson J. Platelet-derived growth factor-induced Akt phosphorylation requires mTOR/Rictor and phospholipase C-γ1, whereas S6 phosphorylation depends on mTOR/Raptor and phospholipase D. Cell Commun Signal 2013; 11(1): 3, https://doi.org/10.1186/1478-811X-11-3.
- Ostendorp T., Diez J., Heizmann C.W., Fritz G. The crystal structures of human S100B in the zinc- and calcium-loaded state at three pH values reveal zinc ligand swapping. Biochim Biophys Acta 2011; 1813(5): 1083–1091, https://doi.org/10.1016/j.bbamcr.2010.10.006.
- Wang H., Mao X., Ye L., Cheng H., Dai X. The role of the S100 protein family in glioma. J Cancer 2022; 13(10): 3022–3030, https://doi.org/10.7150/jca.73365.
- Yin Y., Stephen C.W., Luciani M.G., Fåhraeus R. p53 Stability and activity is regulated by Mdm2-mediated induction of alternative p53 translation products. Nat Cell Biol 2002; 4(6): 462–467, https://doi.org/10.1038/ncb801.
- Marcel V., Perrier S., Aoubala M., Ageorges S., Groves M.J., Diot A., Fernandes K., Tauro S., Bourdon J.C. Δ160p53 is a novel N-terminal p53 isoform encoded by Δ133p53 transcript. FEBS Lett 2010; 584(21): 4463–4468, https://doi.org/10.1016/j.febslet.2010.10.005.
- Pienkowski T., Kowalczyk T., Cysewski D., Kretowski A., Ciborowski M. Glioma and post-translational modifications: a complex relationship. Biochim Biophys Acta Rev Cancer 2023; 1878(6): 189009, https://doi.org/10.1016/j.bbcan.2023.189009.
- Perl K., Ushakov K., Pozniak Y., Yizhar-Barnea O., Bhonker Y., Shivatzki S., Geiger T., Avraham K.B., Shamir R. Reduced changes in protein compared to mRNA levels across non-proliferating tissues. BMC Genomics 2017; 18(1): 305, https://doi.org/10.1186/s12864-017-3683-9.
- Plante I. Dimethylbenz(a)anthracene-induced mammary tumorigenesis in mice. Methods Cell Biol 2021; 163: 21–44, https://doi.org/10.1016/bs.mcb.2020.09.003.
- Avtsyn A.P. Old and new concepts in the teaching on preglioma. Arkh Patol 1972, 34(11): 3–11.
- Kucheryavenko A.S., Chernomyrdin N.V., Gavdush A.A., Alekseeva A.I., Nikitin P.V., Dolganova I.N., Karalkin P.A., Khalansky A.S., Spektor I.E., Skorobogatiy M., Tuchin V.V., Zaytsev K.I. Terahertz dielectric spectroscopy and solid immersion microscopy of ex vivo glioma model 101.8: brain tissue heterogeneity. Biomed Opt Express 2021; 12(8): 5272–5289, https://doi.org/10.1364/BOE.432758.
- Dolganova I.N., Aleksandrova P.V., Nikitin P.V., Alekseeva A.I., Chernomyrdin N.V., Musina G.R., Beshplav S.T., Reshetov I.V., Potapov A.A., Kurlov V.N., Tuchin V.V., Zaytsev K.I. Capability of physically reasonable OCT-based differentiation between intact brain tissues, human brain gliomas of different WHO grades, and glioma model 101.8 from rats. Biomed Opt Express 2020; 11(11): 6780–6798, https://doi.org/10.1364/BOE.409692.
- Kiseleva E.B., Yashin K.S., Moiseev A.A., Timofeeva L.B., Kudelkina V.V., Alekseeva A.I., Meshkova S.V., Polozova A.V., Gelikonov G.V., Zagaynova E.V., Gladkova N.D. Optical coefficients as tools for increasing the optical coherence tomography contrast for normal brain visualization and glioblastoma detection. Neurophotonics 2019; 6(3): 035003, https://doi.org/10.1117/1.NPh.6.3.035003.
- Maksimenko O., Malinovskaya J., Shipulo E., Osipova N., Razzhivina V., Arantseva D., Yarovaya O., Mostovaya U., Khalansky A., Fedoseeva V., Alekseeva A., Vanchugova L., Gorshkova M., Kovalenko E., Balabanyan V., Melnikov P., Baklaushev V., Chekhonin V., Kreuter J., Gelperina S. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: towards the pharmaceutical development. Int J Pharm 2019; 572: 118733, https://doi.org/10.1016/j.ijpharm.2019.118733.
- Dzhalilova D.S., Zolotova N.A., Mkhitarov V.A., Kosyreva A.M., Tsvetkov I.S., Khalansky A.S., Alekseeva A.I., Fatkhudinov T.H., Makarova O.V. Morphological and molecular-biological features of glioblastoma progression in tolerant and susceptible to hypoxia Wistar rats. Sci Rep 2023; 13(1): 12694, https://doi.org/10.1038/s41598-023-39914-9.
- Alekseeva A.I., Sentyabreva A.V., Kudelkina V.V., Miroshnichenko E.A., Ikonnikov A.V., Kopantseva E.E., Kosyreva A.M., Fatkhudinov T.K. Rat glioma 101.8 tissue strain: molecular and morphological features. Int J Mol Sci 2025; 26(18): 8992, https://doi.org/10.3390/ijms26188992.